Corrosion of metals- CIE iGCSE Chemistry Notes - New Syllabus
Corrosion of metals for iGCSE Chemistry Notes
Core Syllabus
- State the conditions required for the rusting of iron and steel to form hydrated iron(III) oxide
- State some common barrier methods, including painting, greasing and coating with plastic
- Describe how barrier methods prevent rusting by excluding oxygen or water
Supplement Syllabus
- Describe the use of zinc in galvanising as an example of a barrier method and sacrificial protection
- Explain sacrificial protection in terms of the reactivity series and in terms of electron loss
Rusting of iron and steel
Rusting of iron and steel
Rusting is the corrosion of iron or steel, forming hydrated iron(III) oxide (\( \text{Fe}_2\text{O}_3 \cdot x\text{H}_2\text{O} \)). It occurs when iron reacts with oxygen and water from the environment.
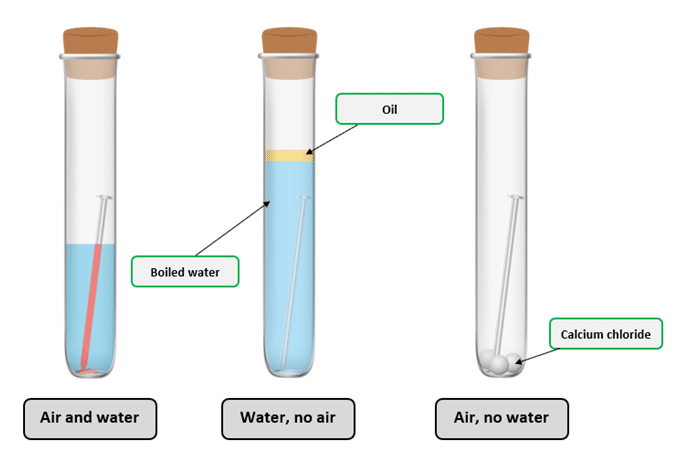
Essential conditions for rusting:
- Presence of water – acts as an electrolyte, allowing ions to move and enabling the redox reactions involved in rusting.
- Presence of oxygen – required to oxidise iron to iron(II) ions and further to iron(III) ions.
- Both water and oxygen are necessary; absence of either one slows or prevents rusting.
Example
Iron nail left in air exposed to moisture gradually becomes covered with reddish-brown flaky rust. Why?
▶️Answer/Explanation
The iron reacts with oxygen and water:
\( 4\text{Fe} + 3\text{O}_2 + 6\text{H}_2\text{O} \rightarrow 4\text{Fe(OH)}_3 \)
On drying, the hydrated iron(III) hydroxide converts to hydrated iron(III) oxide (rust):
\( 4\text{Fe(OH)}_3 \rightarrow 2\text{Fe}_2\text{O}_3 \cdot 3\text{H}_2\text{O} \)
Barrier methods to prevent rusting
Barrier methods to prevent rusting
Barrier methods protect iron and steel from rusting by preventing contact with water and oxygen, which are essential for corrosion. Common methods include:

- Painting – applying a coat of paint to cover the metal surface.
- Greasing – applying oil or grease to form a protective layer.
- Coating with plastic – wrapping or molding the metal in plastic to block exposure.
Example
Iron railings are painted regularly to prevent rusting. How?
▶️Answer/Explanation
The paint forms a protective barrier that prevents water and oxygen from reaching the iron. Without this coating, rust would form on the metal surface.
Example
Cooking utensils are often greased or oiled to prevent corrosion. How?
▶️Answer/Explanation
The oil layer blocks moisture and air from contacting the metal, so rusting is prevented.
Example
Some metal parts are coated with plastic to increase their lifespan. Why?
▶️Answer/Explanation
The plastic coating acts as a waterproof and airtight barrier. Water and oxygen cannot reach the metal, so corrosion is effectively prevented.
How barrier methods prevent rusting
Barrier methods work by stopping water and oxygen from reaching the metal surface, because both are essential for rusting to occur. By creating a physical or chemical barrier, the redox reactions that form rust are blocked.
Mechanism:
- Paint, grease, or plastic coatings physically cover the metal, preventing contact with oxygen and moisture.
- Without water, the electrolyte needed for ion movement is missing, so corrosion reactions cannot proceed.
- Without oxygen, iron cannot oxidize to iron(II) or iron(III) ions, which are required for rust formation.
Example
How does a painted iron gate in a garden prevent it from rusting?
▶️Answer/Explanation
The paint layer prevents air and water from contacting the iron. Even during rain, the iron does not rust because the protective coating blocks oxygen and moisture.
Example
How does a greased metal machinery in a workshop prevent it from rusting?
▶️Answer/Explanation
The layer of grease forms a waterproof barrier, preventing water from reaching the metal. Rusting is prevented even in a humid environment.
Example
How does a plastic-coated steel pipes prevent it from rusting?
▶️Answer/Explanation
The plastic coating completely isolates the metal from air and water. As a result, no rusting occurs even over a long period of exposure.
Use of zinc in galvanising
Use of zinc in galvanising
Galvanising is a method of protecting iron or steel from rusting by coating it with a layer of zinc. Zinc acts both as a barrier and provides sacrificial protection because it is more reactive than iron.
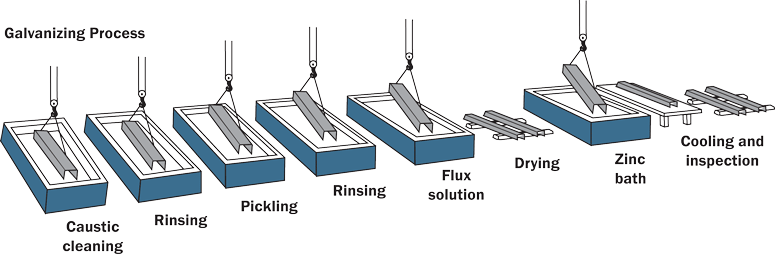
How galvanising works:
- The iron or steel surface is coated with zinc, either by dipping it into molten zinc (hot-dip galvanising) or by electroplating.
- The zinc layer physically prevents oxygen and water from reaching the iron, acting as a **barrier**.
- Even if the zinc coating is scratched, the exposed zinc **sacrificially corrodes** in preference to the iron because zinc is more reactive in the reactivity series.
Example
Why is galvanised steel used in outdoor fencing?
▶️Answer/Explanation
The zinc coating protects the steel from rusting by blocking oxygen and water. Even if the coating is scratched, zinc corrodes instead of the underlying steel, extending the life of the fence.
Sacrificial protection in terms of the reactivity series and electron loss
Sacrificial protection is a method to protect iron or steel from rusting by attaching a more reactive metal to it. The more reactive metal corrodes instead of the iron, hence sacrificing itself.
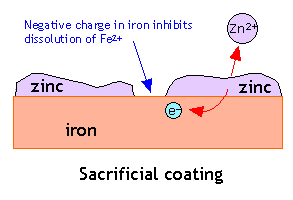
Key points:
- The more reactive metal is higher in the reactivity series than iron (e.g., zinc, magnesium, or aluminium).
- It corrodes preferentially because it loses electrons more easily (oxidation), forming positive ions in place of iron.
- The iron or steel remains unoxidised as long as the sacrificial metal is present and in contact.
- Even if the iron surface is scratched, the sacrificial metal continues to protect it.
Example
Magnesium blocks are used to protect a steel underground pipeline. Why?
▶️Answer/Explanation
Magnesium is more reactive than iron and corrodes preferentially: \( \text{Mg} \rightarrow \text{Mg}^{2+} + 2e^- \) The electrons flow to the iron, preventing it from oxidising. The steel remains protected even if the pipeline coating is damaged.
