Electrolysis- CIE iGCSE Chemistry Notes - New Syllabus
Electrolysis for iGCSE Chemistry Notes
Core Syllabus
- Define electrolysis as the decomposition of an ionic compound, when molten or in aqueous solution, by the passage of an electric current
- Identify in simple electrolytic cells:
(a) the anode as the positive electrode
(b) the cathode as the negative electrode
(c) the electrolyte as the molten or aqueous substance that undergoes electrolysis
Identify the products formed at the electrodes and describe the observations made during the electrolysis of:
(a) molten lead(II) bromide
(b) concentrated aqueous sodium chloride
(c) dilute sulfuric acid using inert electrodes made of platinum or carbon / graphite - State that metals or hydrogen are formed at the cathode and that non-metals (other than hydrogen) are formed at the anode
- Predict the identity of the products at each electrode for the electrolysis of a binary compound in the molten state
- State that metal objects are electroplated to improve their appearance and resistance to corrosion
- Describe how metals are electroplated
Supplement Syllabus
- Describe the transfer of charge during electrolysis to include:
(a) the movement of electrons in the external circuit
(b) the loss or gain of electrons at the electrodes
(c) the movement of ions in the electrolyte - Identify the products formed at the electrodes and describe the observations made during the electrolysis of aqueous copper(II) sulfate using inert carbon / graphite electrodes and when using copper electrodes
- Predict the identity of the products at each electrode for the electrolysis of a halide compound in dilute or concentrated aqueous solution
- Construct ionic half-equations for reactions at the anode (to show oxidation) and at the cathode (to show reduction)
Electrolysis
Electrolysis
Electrolysis is defined as the decomposition of an ionic compound by the passage of an electric current, when the compound is either molten or in aqueous solution.
Only ionic compounds can undergo electrolysis because they consist of mobile ions when melted or dissolved in water.

Key conditions for electrolysis:
- The compound must be ionic
- It must be in the molten state or dissolved in water (aqueous) so that ions are free to move
- An electric current must be passed through the electrolyte
Why decomposition occurs:
The electric current forces the positive ions (cations) to move to the negative electrode (cathode), where they gain electrons (reduction), and the negative ions (anions) to move to the positive electrode (anode), where they lose electrons (oxidation).
Example
Explain why solid sodium chloride does not conduct electricity, but molten sodium chloride does.
▶️Answer/Explanation
In solid sodium chloride, the ions are held in fixed positions and cannot move, so it cannot conduct electricity.
When molten, the ionic lattice breaks down and ions are free to move. These mobile ions carry the electric current, allowing electrolysis to occur.
Identification of Components in Simple Electrolytic Cells
In a basic electrolytic cell, three main parts must be identified:
(a) Anode – the positive electrode
- Connected to the positive terminal of the power supply
- Attracts negatively charged ions (anions)
- Oxidation occurs here (loss of electrons)
(b) Cathode – the negative electrode
- Connected to the negative terminal of the power supply
- Attracts positively charged ions (cations)
- Reduction occurs here (gain of electrons)
(c) Electrolyte – the ionic compound (molten or aqueous) that undergoes electrolysis
- Contains mobile ions that conduct electricity
- Provides the charged particles needed for redox reactions at electrodes
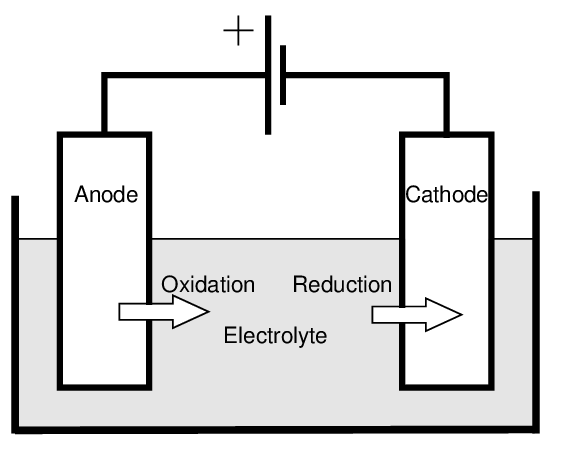
Reminder: The movement of ions in the electrolyte and electrons in the wires allows current to flow and electrolysis to occur.
Example
In the electrolysis of molten potassium bromide using inert electrodes, identify:
- The anode
- The cathode
- The electrolyte
▶️Answer/Explanation
Anode: The positive electrode (connected to + terminal) → bromide ions move here and are oxidized
Cathode: The negative electrode (connected to – terminal) → potassium ions move here and are reduced
Electrolyte: Molten potassium bromide \( (\text{KBr}) \), which provides \( \text{K}^+ \) and \( \text{Br}^- \) ions
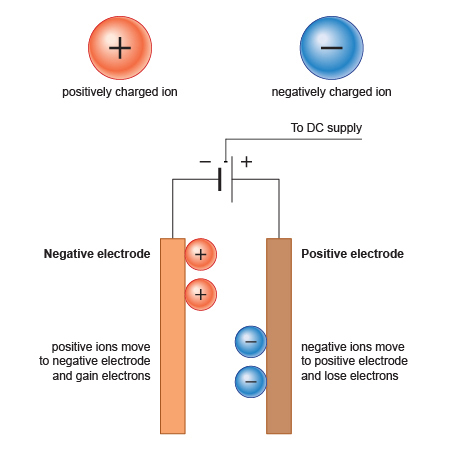
Charge Transfer During Electrolysis
Electrolysis involves the movement of charged particles — electrons and ions — which allows current to flow and chemical reactions to occur at the electrodes. Here’s how the charge is transferred during electrolysis:
(a) Movement of Electrons in the External Circuit
- Electrons flow through the external wires from the anode (positive) to the cathode (negative)
- This flow of electrons is the electric current that drives the redox reactions
(b) Loss or Gain of Electrons at Electrodes
- At the Cathode (−): Reduction occurs — ions gain electrons
e.g. \( \text{Cu}^{2+} + 2e^- \rightarrow \text{Cu} \) - At the Anode (+): Oxidation occurs — ions lose electrons
e.g. \( 2\text{Cl}^- \rightarrow \text{Cl}_2 + 2e^- \)
- At the Cathode (−): Reduction occurs — ions gain electrons
Memory tip: OIL RIG – Oxidation Is Loss (of electrons), Reduction Is Gain (of electrons)
(c) Movement of Ions in the Electrolyte
- Cations (positive ions) move toward the cathode where they are reduced
- Anions (negative ions) move toward the anode where they are oxidized
This completes the circuit inside the solution and allows electrolysis to continue.
Example
Describe how charge is transferred during the electrolysis of molten sodium bromide using graphite electrodes.
▶️Answer/Explanation
Electrons move through wires from the anode to the cathode
At the cathode: \( \text{Na}^+ + e^- \rightarrow \text{Na} \) (reduction)
At the anode: \( 2\text{Br}^- \rightarrow \text{Br}_2 + 2e^- \) (oxidation)
\( \text{Na}^+ \) ions move to the cathode, \( \text{Br}^- \) ions move to the anode in the electrolyte
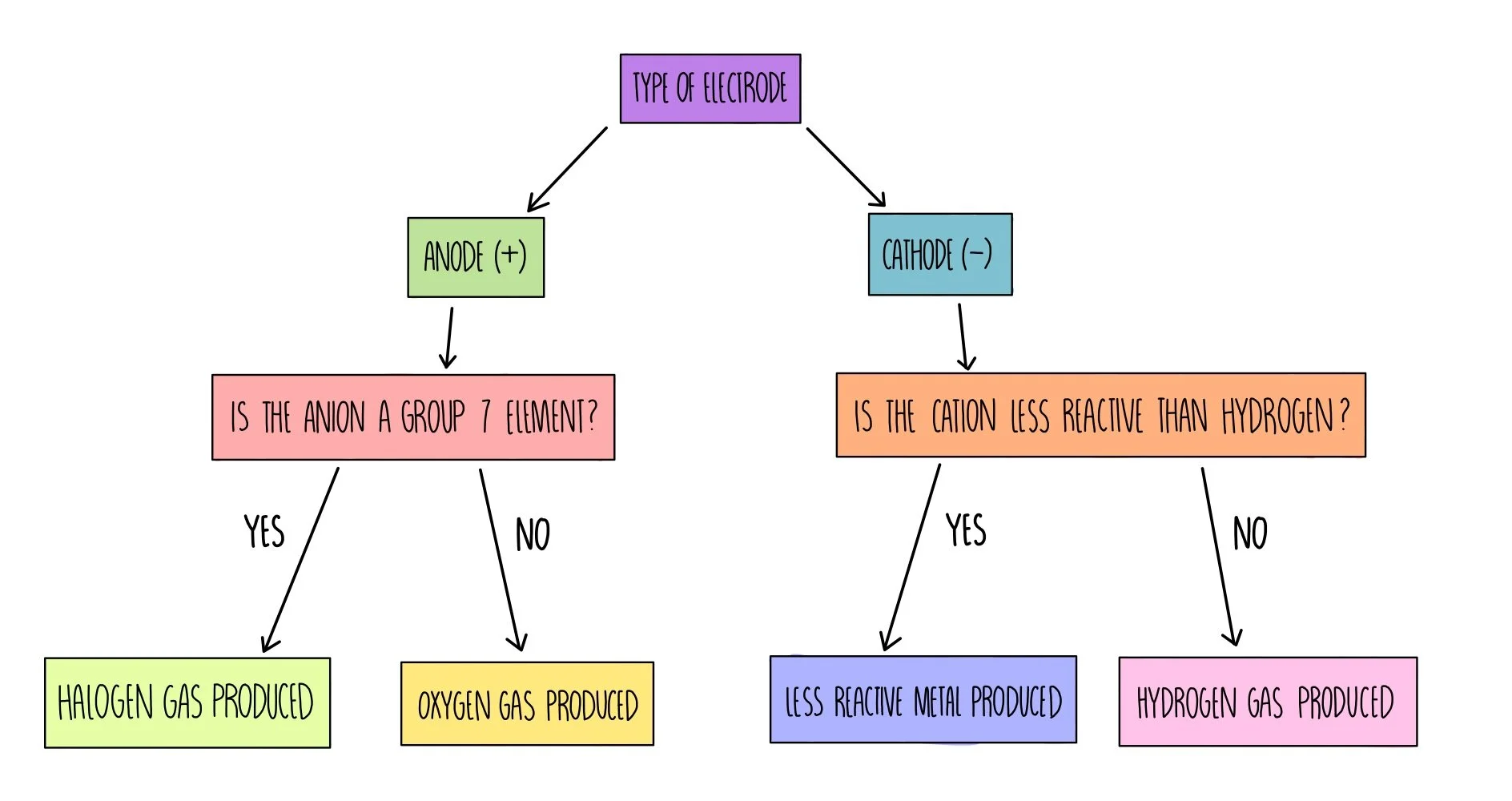
Products at Electrodes During Electrolysis
Rule for Products at Electrodes During Electrolysis
During electrolysis, you can often predict the product at each electrode using these general rules:
- At the Cathode (negative electrode): A metal or hydrogen is produced.
- At the Anode (positive electrode): A non-metal (except hydrogen) is produced.
More specifically:
- If the cation comes from a reactive metal (like sodium, potassium, calcium), then hydrogen gas is produced at the cathode (from the reduction of water).
- If the cation comes from a less reactive metal (like copper, silver), then the metal itself is deposited at the cathode.
- At the anode:
- If the solution contains halide ions (Cl–, Br–, I–), a halogen is formed.
- If the solution contains no halide ions, then oxygen gas is formed (from OH– ions).
Note: These rules apply especially to aqueous solutions where water can be involved in the reactions.
Constructing Ionic Half-Equations at the Electrodes
- Write the ion involved
- Determine whether it gains or loses electrons
- Balance the number of atoms and charges
Examples
- Reduction at Cathode:
- \( \text{Cu}^{2+} + 2e^- \rightarrow \text{Cu} \)
- \( 2\text{H}^+ + 2e^- \rightarrow \text{H}_2 \)
- Oxidation at Anode:
- \( 2\text{Cl}^- \rightarrow \text{Cl}_2 + 2e^- \)
- \( 4\text{OH}^- \rightarrow \text{O}_2 + 2\text{H}_2\text{O} + 4e^- \)
- Reduction at Cathode:
Tip: Make sure the number of electrons lost or gained balances the charges on both sides.
Example
Write the ionic half-equations for the electrolysis of molten aluminum oxide \( (\text{Al}_2\text{O}_3) \).
▶️Answer/Explanation
Molten Al₂O₃ → contains \( \text{Al}^{3+} \) and \( \text{O}^{2-} \) ions
Cathode (reduction): \( \text{Al}^{3+} + 3e^- \rightarrow \text{Al} \)
Anode (oxidation): \( 2\text{O}^{2-} \rightarrow \text{O}_2 + 4e^- \)
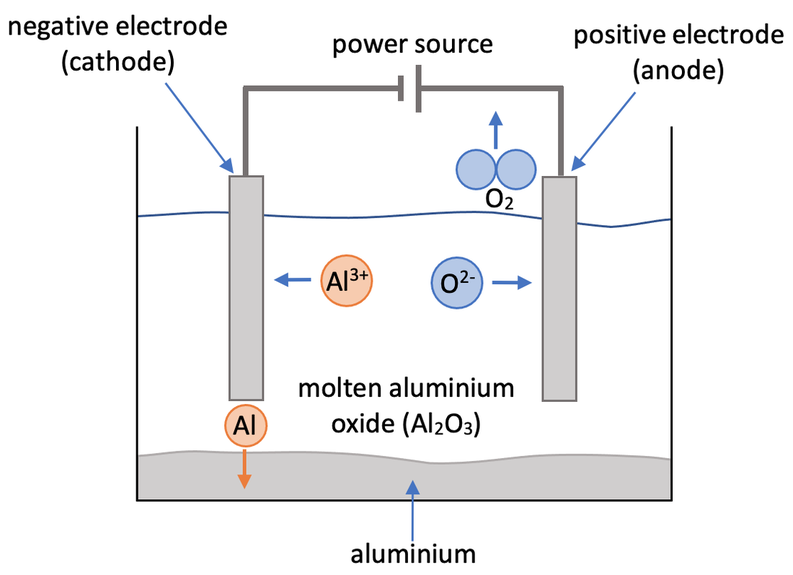
Predicting Products of Electrolysis for Binary Molten Compounds
A binary compound contains only two elements. When such a compound is in the molten state, it breaks into its ions and can undergo electrolysis.
General Rule:
- The metal cation is reduced at the cathode to form the metal.
- The non-metal anion is oxidised at the anode to form the non-metal element.
Example: Magnesium chloride \( (\text{MgCl}_2) \)
When molten, it contains \( \text{Mg}^{2+} \) and \( \text{Cl}^- \)
- Cathode (-): \( \text{Mg}^{2+} + 2e^- \rightarrow \text{Mg} \) (solid magnesium formed)
- Anode (+): \( 2\text{Cl}^- \rightarrow \text{Cl}_2 + 2e^- \) (chlorine gas released)
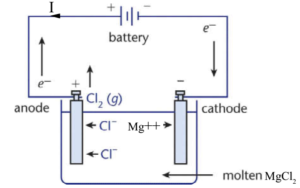
Prediction Steps:
- Write the ions present in the molten compound.
- Cations go to the cathode and are reduced.
- Anions go to the anode and are oxidised.
Example
Predict the products formed during the electrolysis of molten calcium iodide using inert electrodes.
▶️Answer/Explanation
Ions present: \( \text{Ca}^{2+} \) and \( \text{I}^- \)
At cathode: \( \text{Ca}^{2+} + 2e^- \rightarrow \text{Ca} \) → calcium metal
At anode: \( 2\text{I}^- \rightarrow \text{I}_2 + 2e^- \) → iodine gas
Observation: Silvery calcium forms at cathode; purple iodine vapour at anode
Predicting Products During Electrolysis of Halide Solutions
When electrolyzing dilute or concentrated aqueous solutions of halide compounds (e.g. sodium chloride, potassium bromide), the products at the electrodes depend on:
- Which ions are present
- Whether the solution is dilute or concentrated
Ions present in aqueous halide solutions:
- From salt: a metal cation (e.g. \( \text{Na}^+ \), \( \text{K}^+ \)) and a halide anion (e.g. \( \text{Cl}^- \), \( \text{Br}^- \))
- From water: \( \text{H}^+ \) and \( \text{OH}^- \)
At the Cathode (negative electrode):
- Hydrogen gas is formed if the metal is more reactive than hydrogen (like Na, K, Ca)
- \( 2\text{H}^+ + 2e^- \rightarrow \text{H}_2 \)
At the Anode (positive electrode):
- In concentrated solution: the halide ion is discharged
- In dilute solution: the hydroxide ion (OH⁻) is discharged instead
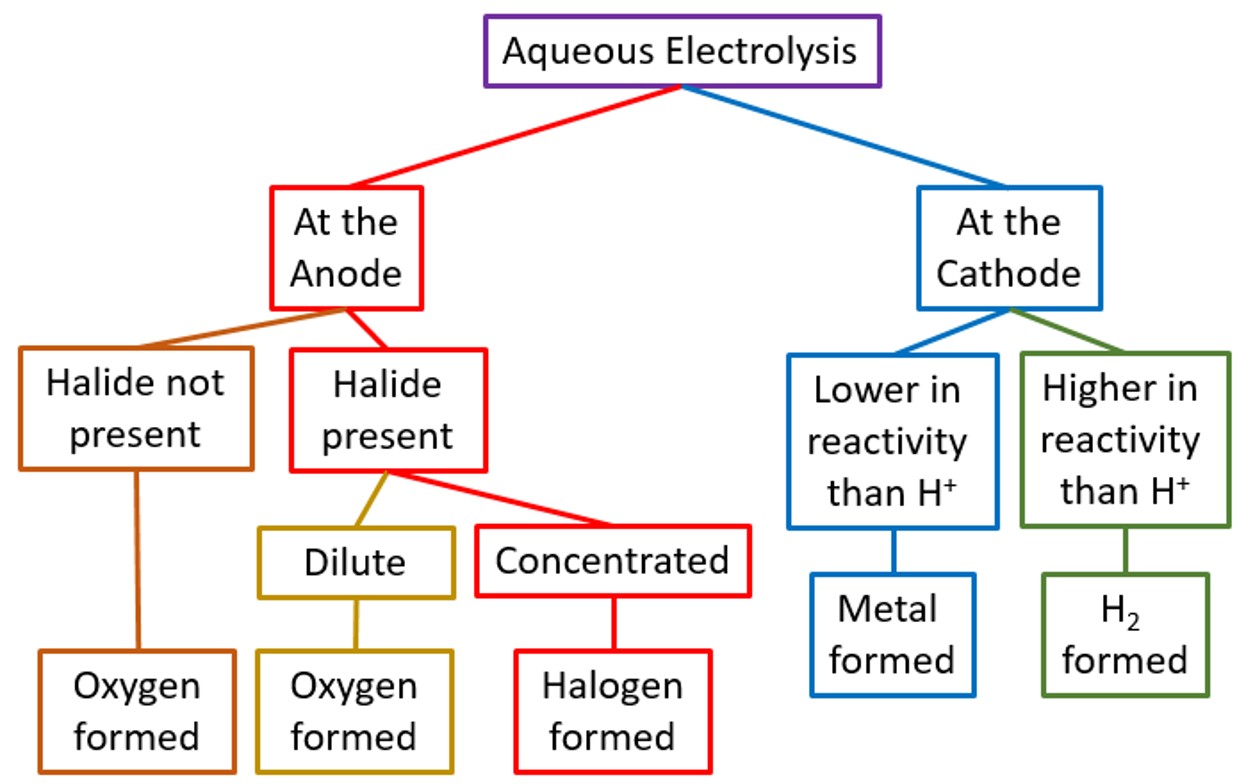
| Solution | Cathode Product | Anode Product |
|---|---|---|
| Dilute NaCl (aq) | Hydrogen gas | Oxygen gas |
| Concentrated NaCl (aq) | Hydrogen gas | Chlorine gas |
| Concentrated KBr (aq) | Hydrogen gas | Bromine gas |
Example
Predict the products at each electrode during the electrolysis of dilute aqueous sodium chloride.
▶️Answer/Explanation
Ions present: \( \text{Na}^+ \), \( \text{Cl}^- \), \( \text{H}^+ \), \( \text{OH}^- \)
Cathode: \( 2\text{H}^+ + 2e^- \rightarrow \text{H}_2 \) → hydrogen gas
Anode: \( 4\text{OH}^- \rightarrow \text{O}_2 + 2\text{H}_2\text{O} + 4e^- \) → oxygen gas
Observation: Bubbles at both electrodes; test gases with a lit splint (hydrogen pops) and glowing splint (oxygen relights)
Identify the Products Formed at the Electrodes and Describe the Observations During Electrolysis
You should be able to identify the substances formed at the anode and cathode, and describe what you would observe during electrolysis of:
- Molten lead(II) bromide
- Concentrated aqueous sodium chloride
- Dilute sulfuric acid
Key Note: These processes use inert electrodes (graphite or platinum), which do not react themselves.
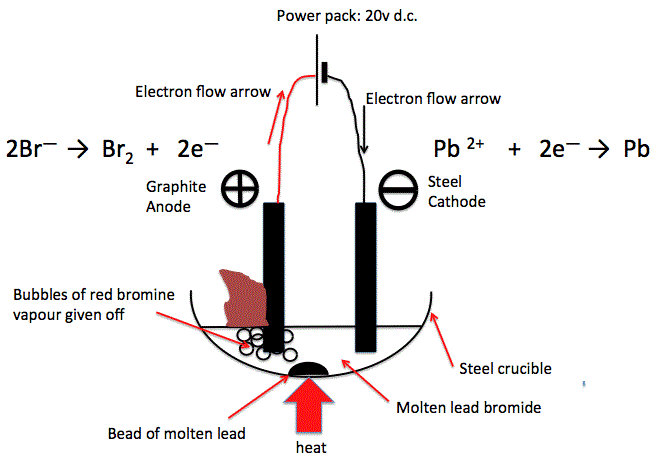
(a) Electrolysis of Molten Lead(II) Bromide (\( \text{PbBr}_2 \))
- Cathode (-): \( \text{Pb}^{2+} + 2e^- \rightarrow \text{Pb} \) (grey metal forms)
- Anode (+): \( 2\text{Br}^- \rightarrow \text{Br}_2 + 2e^- \) (brown vapour of bromine)
Observation: Shiny lead metal forms at the cathode, brown fumes (bromine gas) at the anode.
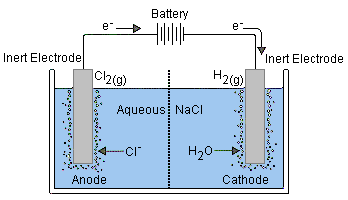
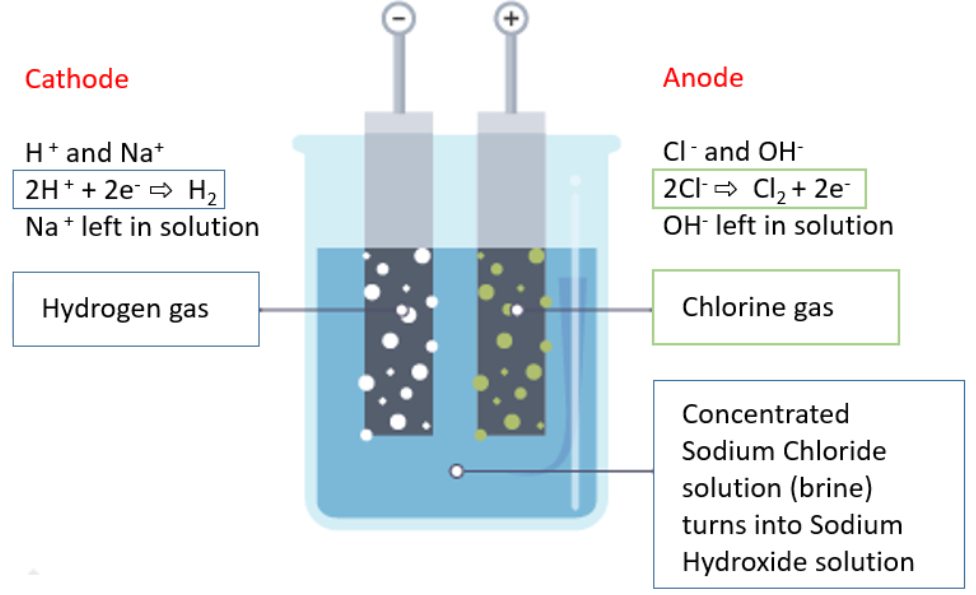
(b) Electrolysis of Concentrated Aqueous Sodium Chloride (\( \text{NaCl}_{(aq)} \))
Contains \( \text{Na}^+ \), \( \text{Cl}^- \), \( \text{H}^+ \), \( \text{OH}^- \)
- Cathode (-): \( 2\text{H}^+ + 2e^- \rightarrow \text{H}_2 \) (bubbles of hydrogen gas)
- Anode (+): \( 2\text{Cl}^- \rightarrow \text{Cl}_2 + 2e^- \) (greenish-yellow chlorine gas)
Observation: Effervescence at both electrodes. Chlorine gas smells pungent and turns damp blue litmus paper red, then bleaches it.
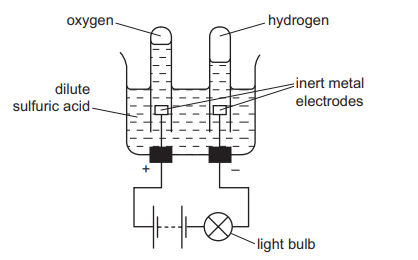
(c) Electrolysis of Dilute Sulfuric Acid (\( \text{H}_{2}\text{SO}_{4\text{(aq)}} \))
Contains \( \text{H}^+ \), \( \text{SO}_4^{2-} \), \( \text{OH}^- \)
- Cathode (-): \( 2\text{H}^+ + 2e^- \rightarrow \text{H}_2 \)
- Anode (+): \( 4\text{OH}^- \rightarrow \text{O}_2 + 2\text{H}_2\text{O} + 4e^- \)
Observation: Bubbles of hydrogen at the cathode; colourless oxygen at the anode. Glowing splint relights in oxygen.
Example
What would you observe at each electrode during the electrolysis of dilute sulfuric acid using carbon electrodes?
▶️Answer/Explanation
At the cathode: bubbles of hydrogen gas form
At the anode: bubbles of oxygen gas form
Test: Hydrogen pops with a lit splint; oxygen relights a glowing splint
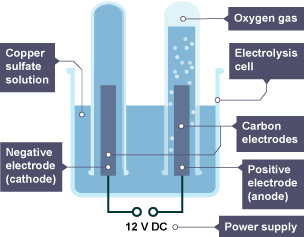
Electrolysis of Aqueous Copper(II) Sulfate with Different Electrodes
Case 1: Using Inert Electrodes (Carbon/Graphite)
Ions present: \( \text{Cu}^{2+} \), \( \text{SO}_4^{2-} \), \( \text{H}^+ \), \( \text{OH}^- \)
- Cathode (-): \( \text{Cu}^{2+} + 2e^- \rightarrow \text{Cu} \)
Copper metal is deposited (reddish-brown layer)
- Anode (+): \( 4\text{OH}^- \rightarrow \text{O}_2 + 2\text{H}_2\text{O} + 4e^- \)
Oxygen gas is produced (colourless bubbles)
Observations: Brown copper coats the cathode, oxygen bubbles at the anode. Solution gradually becomes more acidic due to remaining \( \text{H}^+ \) ions.
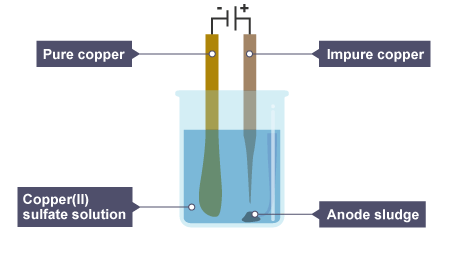
Case 2: Using Copper Electrodes
What changes: The copper anode is no longer inert – it participates in the reaction.
- Cathode (-): \( \text{Cu}^{2+} + 2e^- \rightarrow \text{Cu} \)
Copper ions from solution are reduced and deposited on cathode
- Anode (+): \( \text{Cu} \rightarrow \text{Cu}^{2+} + 2e^- \)
Copper metal from the anode dissolves into solution
Observations: Copper anode gradually gets thinner, copper builds up on cathode. No change in blue colour of solution (copper ion concentration stays constant).
Used in: Electrorefining of copper — to purify copper for industrial use.
Example
What would you observe when aqueous copper(II) sulfate is electrolysed using carbon electrodes?
▶️Answer/Explanation
Ions present: \( \text{Cu}^{2+} \), \( \text{SO}_4^{2-} \), \( \text{H}^+ \), \( \text{OH}^- \)
At the cathode: \( \text{Cu}^{2+} + 2e^- \rightarrow \text{Cu} \) → copper metal is deposited
At the anode: \( 4\text{OH}^- \rightarrow \text{O}_2 + 2\text{H}_2\text{O} + 4e^- \) → oxygen gas is released
Observation: The blue colour of the solution fades slowly as \( \text{Cu}^{2+} \) ions are removed. Reddish-brown copper coats the cathode; colourless gas bubbles at the anode (glowing splint relights).
Electroplating
Electroplating
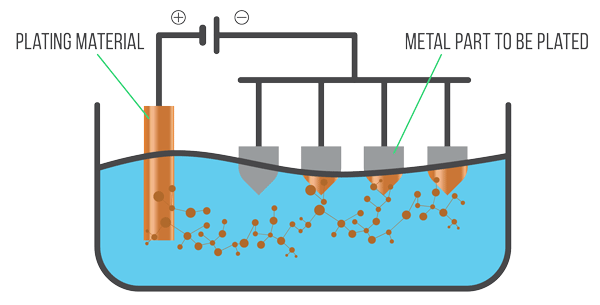
Electroplating is the process of depositing a thin layer of metal onto the surface of another material using electrolysis.
Purposes of Electroplating:
- To improve appearance: e.g. silver plating on jewellery to make it look attractive
- To prevent corrosion: e.g. coating iron with a layer of nickel or chromium to protect against rust
- To reduce friction: in machinery and moving parts
- To increase hardness/resistance: to wear or chemical attack
How Electroplating Works:
The object to be plated and the metal to be deposited are placed in an electrolytic cell containing a suitable electrolyte.
- Cathode (−): The object that is to be plated (e.g. a steel spoon)
- Anode (+): A piece of the pure metal to be used for plating (e.g. silver or copper)
- Electrolyte: A solution containing metal ions of the plating metal (e.g. silver nitrate for silver plating)
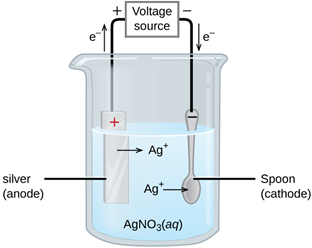
Example Setup:
To electroplate a steel spoon with silver:
- Cathode: Steel spoon (object being plated)
- Anode: Pure silver rod
- Electrolyte: Aqueous silver nitrate solution
Overall reaction: Silver from the anode dissolves and is deposited on the spoon.
How Are Metals Electroplated?
Electroplating is carried out using an electrolytic cell. This involves an external power supply, two electrodes, and an electrolyte containing ions of the metal to be deposited.
Step-by-Step Process of Electroplating:
1. Clean the metal object (to be electroplated):
- Any dirt, grease, or oxide layer must be removed so that the coating adheres properly.
2. Set up the electrolytic cell:
- Place the object to be plated as the cathode (negative electrode)
- Use a rod of the plating metal as the anode (positive electrode)
- Immerse both in an electrolyte that contains ions of the plating metal
- Connect to a power supply:
- Connect the cathode to the negative terminal and the anode to the positive terminal
3. Electrolysis occurs:
- At the cathode, metal ions from the electrolyte are reduced and deposited onto the object:
\( \text{M}^+ + e^- \rightarrow \text{M} \)
- At the anode, atoms of the metal are oxidised to release metal ions into the solution:
\( \text{M} \rightarrow \text{M}^+ + e^- \)
Example:
- \( \text{Ag}^+ + e^- \rightarrow \text{Ag} \) (plated onto spoon)
- \( \text{Ag} \rightarrow \text{Ag}^+ + e^- \)
4. Result:
- A uniform metal coating is formed on the object
- The concentration of metal ions in the electrolyte remains constant
Key Points to Remember:
- The metal to be electroplated is always the cathode
- The metal used for plating is the anode
- The electrolyte must contain ions of the plating metal
- Electroplating only works when the electrolyte is aqueous or molten
Example
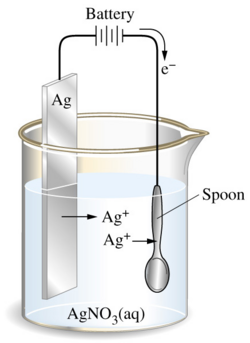
Explain how a copper spoon can be electroplated with silver and state the role of each part in the process.
▶️Answer/Explanation
Connect the copper spoon to the cathode (negative terminal).
Use a silver rod as the anode (positive terminal).
Immerse both electrodes in aqueous silver nitrate solution.
Silver ions \( \text{Ag}^+ \) in the solution gain electrons at the cathode:
\( \text{Ag}^+ + e^- \rightarrow \text{Ag} \) → silver coats the spoon.
At the anode, silver metal is oxidised to release more silver ions:
\( \text{Ag} \rightarrow \text{Ag}^+ + e^- \)
Example
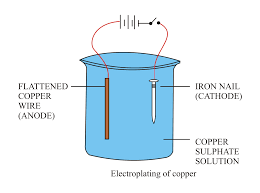
Describe how to electroplate an iron nail with copper. Identify all key components in the cell.
▶️Answer/Explanation
Step 1: Preparation
Clean the iron nail thoroughly to remove any grease, rust, or dirt. This ensures proper adhesion of copper.
Step 2: Setting up the circuit
• Connect the iron nail to the negative terminal of the power supply – it acts as the cathode.
• Use a copper rod as the anode – connect it to the positive terminal of the power supply.
• Use aqueous copper(II) sulfate solution as the electrolyte.
Step 3: Electrolysis process
During electrolysis:
- At the cathode (iron nail): \( \text{Cu}^{2+} + 2e^- \rightarrow \text{Cu} \)
Copper ions gain electrons and get deposited on the nail. - At the anode (copper rod): \( \text{Cu} \rightarrow \text{Cu}^{2+} + 2e^- \)
Copper from the anode dissolves into the solution to replace the ions used up.
As a result, the iron nail becomes coated with a thin layer of copper, while the copper anode slowly dissolves to maintain the concentration of copper ions in the solution.