Extraction of metals- CIE iGCSE Chemistry Notes - New Syllabus
Extraction of metals for iGCSE Chemistry Notes
Core Syllabus
Describe the ease in obtaining metals from their ores, related to the position of the metal in the reactivity series
Describe the extraction of iron from hematite in the blast furnace, limited to:
(a) the burning of carbon (coke) to provide heat and produce carbon dioxide
(b) the reduction of carbon dioxide to carbon monoxide
(c) the reduction of iron(III) oxide by carbon monoxide
(d) the thermal decomposition of calcium carbonate / limestone to produce calcium oxide
(e) the formation of slag
Symbol equations are not requiredState that the main ore of aluminium is bauxite and that aluminium is extracted by electrolysis
Supplement Syllabus
State the symbol equations for the extraction of iron from hematite
(a) C + O2 → CO2
(b) C + CO2 → 2CO
(c) Fe2O3 + 3CO → 2Fe + 3CO2
(d) CaCO3 → CaO + CO2
(e) CaO + SiO2 → CaSiO2Describe the extraction of aluminium from purified bauxite / aluminium oxide, including:
(a) the role of cryolite
(b) why the carbon anodes need to be regularly replaced
(c) the reactions at the electrodes, including ionic half-equations
Details of the purification of bauxite are not required
Obtaining metals from their ores
Obtaining metals from their ores
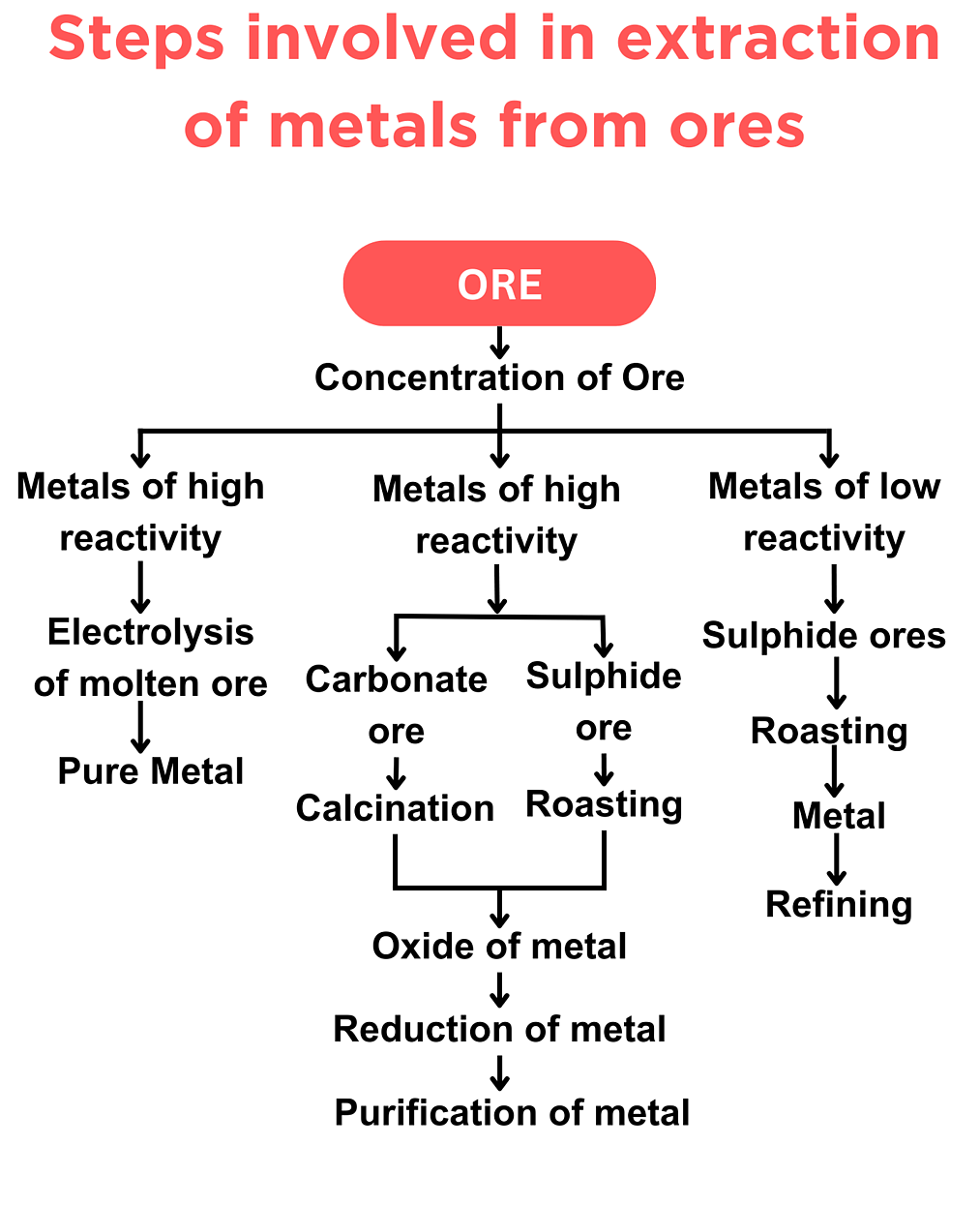
The ease of extracting a metal from its ore depends on how reactive the metal is. The more reactive the metal, the more difficult it is to extract from its compound.
Key Points:
- Metals that are low in the reactivity series (e.g., gold, silver, copper) are often found in their native (uncombined) state. They do not react easily with oxygen or water, so they can be used directly or extracted easily using simple methods.
- Metals of medium reactivity (e.g., iron, zinc, tin) are usually found as oxides or sulfides. They need chemical reduction with carbon or carbon monoxide to extract the metal.
- Metals high in the reactivity series (e.g., aluminium, magnesium, sodium, potassium) are very reactive and form stable compounds. They cannot be reduced by carbon and require electrolysis to extract the metal.
- The higher a metal is in the reactivity series, the more energy-intensive and expensive its extraction process is.
Example
Compare the extraction methods for iron and aluminium and explain why they differ.
▶️Answer/Explanation
- Iron is a moderately reactive metal and is extracted from hematite (Fe2O3) by reduction with carbon in a blast furnace.
- Aluminium is a very reactive metal and cannot be reduced by carbon. It is extracted from bauxite (Al2O3) by electrolysis.
- The difference in methods is due to their positions in the reactivity series: aluminium is higher than iron, making it more difficult to reduce chemically.
Example
Explain why gold can often be found as a native metal while sodium cannot.
▶️Answer/Explanation
- Gold is very unreactive (low in the reactivity series) and does not easily form compounds, so it can be found in its native state.
- Sodium is highly reactive (high in the reactivity series) and forms stable compounds with oxygen and water, so it is never found as a native metal.
Extraction of iron from hematite in the blast furnace
Extraction of iron from hematite in the blast furnace
Iron is extracted from its ore, hematite (Fe2O3), in a blast furnace. The process involves several steps, each with a specific function to ensure efficient production of molten iron.
Key Steps and Explanations: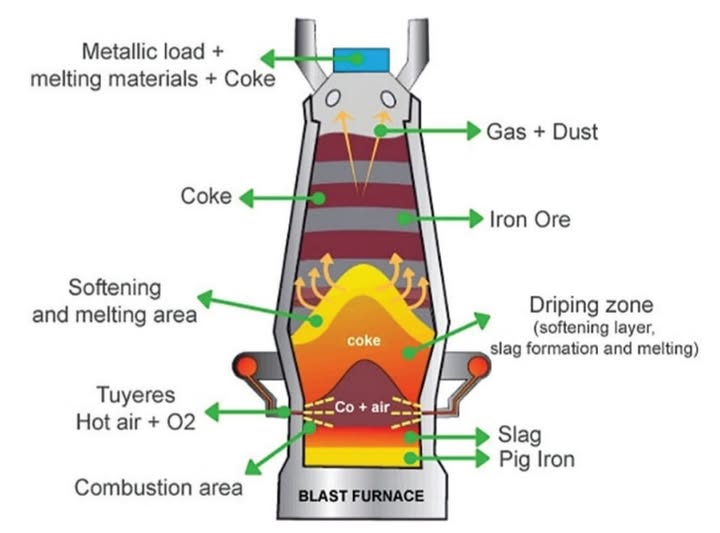
Burning of carbon (coke):
Coke, a form of carbon, is burned in the presence of hot air to provide the high temperatures needed for the blast furnace (about 1500°C).
Reaction: \( \text{C} + \text{O}_2 \rightarrow \text{CO}_2 \)
- This reaction generates the high temperatures (about 1500°C) necessary for the blast furnace to operate.
- The heat melts iron and facilitates the reduction reactions.
- Without sufficient heat, reduction of iron ore would be inefficient.
Reduction of carbon dioxide to carbon monoxide:
The carbon dioxide produced reacts with more coke to form carbon monoxide, which is the main reducing agent in the furnace.
Reaction: \( \text{C} + \text{CO}_2 \rightarrow 2\text{CO} \)
- Carbon monoxide can reduce iron(III) oxide to iron.
- This step ensures that the reduction process continues efficiently at high temperatures without unwanted by-products.
Reduction of iron(III) oxide:
Carbon monoxide reduces hematite (Fe2O3)) to molten iron.
Reaction: \( \text{Fe}_2\text{O}_3 + 3\text{CO} \rightarrow 2\text{Fe} + 3\text{CO}_2 \)
- This process separates iron from oxygen, converting it from an oxide to the pure metal.
- Molten iron collects at the bottom of the furnace for tapping.
Thermal decomposition of limestone:
Limestone (CaCO3) decomposes to produce calcium oxide and carbon dioxide.
Reaction: \( \text{CaCO}_3 \rightarrow \text{CaO} + \text{CO}_2 \)
- Calcium oxide acts as a flux, combining with impurities.
- This step is essential to remove silicate impurities (sand) from the ore.
- Without this step, the iron would be contaminated by sand and other silicates.
Formation of slag: Calcium oxide reacts with silicon dioxide (SiO2) impurities to form calcium silicate (slag).
Reaction: \( \text{CaO} + \text{SiO}_2 \rightarrow \text{CaSiO}_3 \)
- Slag is lighter than molten iron and floats on top, making it easy to remove.
- It protects the molten iron from oxidation and removes impurities, improving the quality of the iron.
Example
Explain why carbon monoxide is used to reduce iron(III) oxide instead of carbon directly in the blast furnace.
▶️Answer/Explanation
- Carbon monoxide is more effective as a reducing agent at the high temperatures of the blast furnace.
- It reacts directly with iron(III) oxide to produce molten iron and carbon dioxide.
- Using carbon directly would be less efficient and produce more unwanted side products.
- Carbon monoxide also allows the furnace to operate continuously without interruptions.
Example
Explain the purpose of limestone and slag formation in iron extraction.
▶️Answer/Explanation
- Limestone decomposes to form calcium oxide, which reacts with silicon dioxide impurities to form calcium silicate (slag).
- Slag floats on molten iron, preventing contamination and oxidation of iron.
- This ensures the molten iron is purer and stronger, suitable for industrial applications.
- Slag can also be removed and used in road construction or cement, making the process efficient.
Extraction of Aluminium from Bauxite
Extraction of Aluminium from Bauxite
Aluminium is a highly reactive metal and cannot be extracted from its ore by carbon reduction. Its main ore is bauxite, which contains aluminium oxide (Al2O3) along with impurities such as iron oxide and silica. Aluminium is extracted industrially using electrolysis of molten aluminium oxide.
Detailed Steps:
- Bauxite is treated to remove impurities such as iron oxide and silica.
- The purified product is mainly aluminium oxide (Al2O3), which is the starting material for electrolysis.
- Details of the Bayer process (used for purification) are not required at IGCSE level, but it ensures that only Al2O3 enters the electrolysis step.
Role of cryolite:
- Pure aluminium oxide has a very high melting point (~2072°C), making direct electrolysis impractical.
- It is dissolved in molten cryolite (Na3AlF6), which lowers the melting point to around 900°C.
- This reduces energy consumption and allows the electrolysis to occur at industrially feasible temperatures.
Electrodes:
- The electrolytic cell has a carbon-lined cathode and carbon anodes.
- Molten aluminium forms at the cathode, while oxygen is produced at the anode.
- Carbon is used because it conducts electricity and can withstand high temperatures.
Electrolysis process:
- The electrolysis cell has a carbon-lined cathode and carbon anodes.
- At the cathode, aluminium ions are reduced to form molten aluminium (reduction):
\( \text{Al}^{3+} + 3e^- \rightarrow \text{Al} \)
- At the anode, oxide ions are oxidized to form oxygen gas (oxidation):
\( 2\text{O}^{2-} \rightarrow \text{O}_2 + 4e^- \)
- The oxygen reacts with the carbon anode to produce CO2:
\( \text{C} + \text{O}_2 \rightarrow \text{CO}_2 \)
Replacement of carbon anodes:
- Carbon anodes are gradually consumed as oxygen reacts with them to form CO2.
- Anodes must be regularly replaced to maintain the electrolysis reaction.
- Failure to replace the anodes stops the electrolysis reaction, interrupting aluminium production.
Example
Explain why aluminium is extracted by electrolysis and not by reduction with carbon.
▶️Answer/Explanation
- Aluminium is highly reactive and forms very stable oxide compounds.
- Carbon cannot reduce aluminium oxide to aluminium.
- Electrolysis uses electric current to separate aluminium from oxygen, making it the only practical method for industrial extraction.
Example
Write the reactions that occur at the cathode and anode during aluminium extraction.
▶️Answer/Explanation
- Cathode: \( \text{Al}^{3+} + 3e^- \rightarrow \text{Al} \)
- Anode: \( 2\text{O}^{2-} \rightarrow \text{O}_2 + 4e^- \)
- Oxygen produced reacts with carbon anode: \( \text{C} + \text{O}_2 \rightarrow \text{CO}_2 \)
Example
Why is cryolite used in aluminium extraction, and what would happen if it were not used?
▶️Answer/Explanation
- Aluminium oxide has a very high melting point (~2072°C), making direct electrolysis impractical.
- Cryolite lowers the melting point to ~900°C, reducing energy costs.
- Without cryolite, the electrolysis process would require extremely high temperatures, making industrial extraction inefficient and expensive.
