Formulae, functional groups and terminology - CIE iGCSE Chemistry Notes - New Syllabus
Formulae, functional groups and terminology for iGCSE Chemistry Notes
Core Syllabus
- Draw and interpret the displayed formula of a molecule to show all the atoms and all the bonds
- Write and interpret general formulae of compounds in the same homologous series, limited to:
(a) alkanes, CnH2n+2
(b) alkenes, CnH2n
(c) alcohols, CnH2n+1OH
(d) carboxylic acids, CnH2n+1COOH - Identify a functional group as an atom or group of atoms that determine the chemical properties of a homologous series
- State that a homologous series is a family of similar compounds with similar chemical properties due to the presence of the same functional group
- State that a saturated compound has molecules in which all carbon–carbon bonds are single bonds
- State that an unsaturated compound has molecules in which one or more carbon–carbon bonds are not single bonds
Supplement Syllabus
- State that a structural formula is an unambiguous description of the way the atoms in a molecule are arranged, including CH2=CH2, CH3CH2OH, CH3COOCH3
- Define structural isomers as compounds with the same molecular formula, but different structural formulae, including C4H10 as CH3CH2CH2CH3 and CH3CH(CH3)CH3 and C4H8 as CH3CH2CH=CH2 and CH3CH=CHCH3
Describe the general characteristics of a homologous series as:
(a) having the same functional group
(b) having the same general formula
(c) differing from one member to the next by a –CH2– unit
(d) displaying a trend in physical properties
(e) sharing similar chemical properties
Saturated Compounds and Unsaturated Compounds
Saturated Compounds
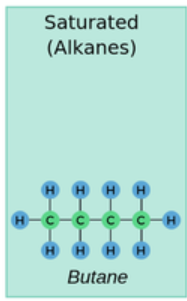
- A saturated compound is one in which all the carbon–carbon bonds are single bonds. This means that every carbon atom is bonded to as many hydrogen atoms as possible.
- In other words, the molecule has the maximum number of hydrogen atoms that the carbon skeleton can accommodate.
Key features:
- Only single covalent bonds (C–C) are present between carbon atoms
- Belong mainly to the family of alkanes
- They are generally less reactive compared to unsaturated compounds, since single bonds are relatively stable
- They burn in air to produce carbon dioxide and water, provided oxygen is sufficient
Examples:
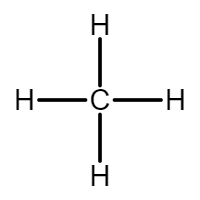
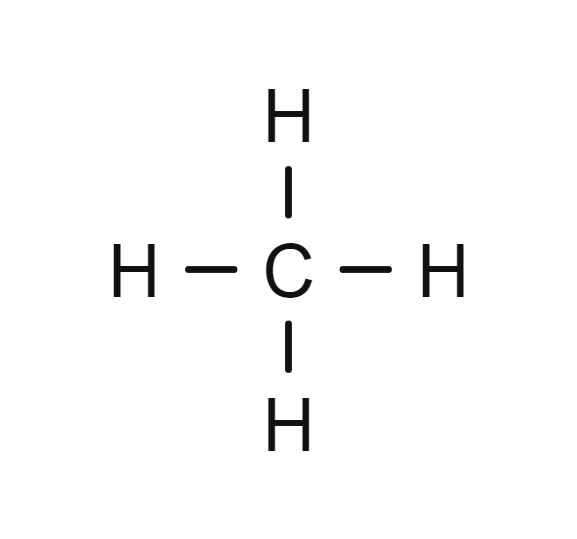
- Methane (\( \text{CH}_4 \)) – the simplest saturated compound
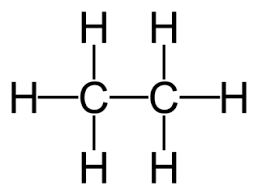
- Ethane (\( \text{C}_2\text{H}_6 \)) – all C–C and C–H bonds are single
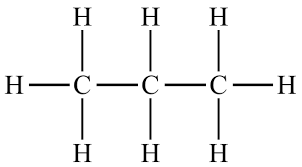
- Propane (\( \text{C}_3\text{H}_8 \)) – again, all carbon atoms connected by single bonds only
Example
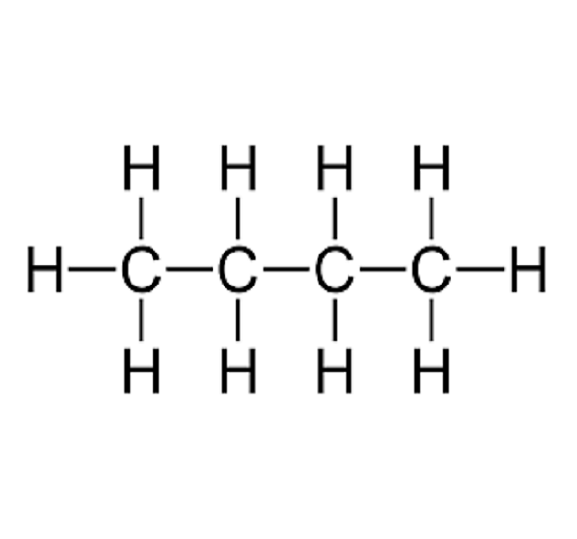
State whether butane (\( \text{C}_4\text{H}_{10} \)) is a saturated compound.
▶️Answer/Explanation
Yes. Butane is an alkane. Its displayed formula shows only C–C single bonds and C–H single bonds, meaning it is fully saturated with hydrogen atoms.
Unsaturated Compounds
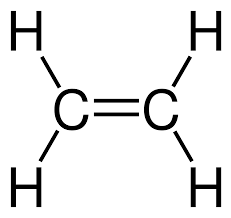
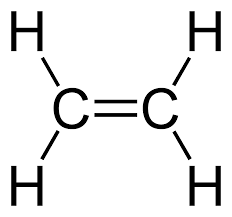
An unsaturated compound is one in which the molecule contains at least one carbon–carbon double bond (C=C) or triple bond (C≡C). Because of these multiple bonds, such compounds are not holding the maximum possible number of hydrogen atoms.
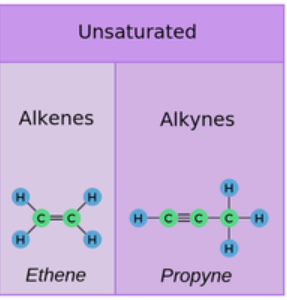
Key features:
- Presence of C=C double bond or C≡C triple bond
- Belong mainly to the families of alkenes and alkynes
- More reactive than saturated compounds because the double and triple bonds are regions of high electron density
- Can undergo addition reactions (such as hydrogenation, halogenation, hydration)
- Burn in air but often with a smoky flame due to higher carbon content
Examples:
- Propene (\( \text{C}_3\text{H}_6 \)) – also contains a double bond
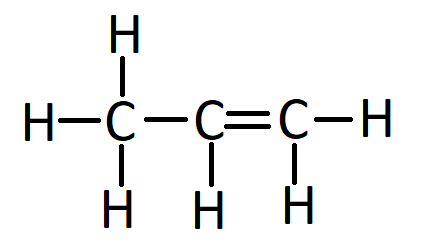
- Ethyne (\( \text{C}_2\text{H}_2 \)) – contains a C≡C triple bond
![]()
Example
State whether ethene (\( \text{C}_2\text{H}_4 \)) is a saturated or unsaturated compound.
▶️Answer/Explanation
Ethene is unsaturated. Its displayed formula shows a carbon–carbon double bond (C=C), meaning it does not contain only single bonds and can undergo addition reactions.
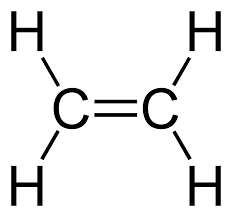
Displayed Formula
Displayed Formula
The displayed formula of a molecule is a structural representation that shows all the atoms and all the covalent bonds between them. It makes the bonding in a molecule clear by drawing each bond as a line between the relevant atoms.
Key features:
- Every atom in the molecule is shown explicitly
- Each covalent bond is represented by a line (single, double, or triple)
- Lone pairs are usually not shown at this level
- Gives an unambiguous picture of how atoms are joined together
Examples:
- Methane (\( \text{CH}_4 \)) – each H atom bonded to C with a single line

- Ethene (\( \text{C}_2\text{H}_4 \)) – shows a C=C double bond

- Water (\( \text{H}_2\text{O} \)) – shows O atom bonded to two H atoms with single bonds

Example
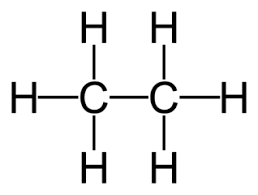
Draw the displayed formula of ethane (\( \text{C}_2\text{H}_6 \)).
▶️Answer/Explanation
Ethane consists of two carbon atoms joined by a single bond.
Each carbon is bonded to enough hydrogen atoms to complete four bonds in total with each carbon also bonded to three hydrogens.
Functional Group
Functional Group
A functional group is an atom or a group of atoms within an organic molecule that determines its characteristic chemical properties. Molecules belonging to the same homologous series share the same functional group, which gives them similar reactivity and chemical behaviour.
Key ideas:
- The functional group is the most reactive part of the molecule
- It defines the type of organic compound (alkane, alkene, alcohol, carboxylic acid, etc.)
- Compounds with the same functional group show similar chemical reactions
Common functional groups:
- Alkanes – no functional group (only single bonds)
- Alkenes – C=C double bond
- Alcohols – OH group
- Carboxylic acids – COOH group
Example
Identify the functional group in ethanol (\( \text{C}_2\text{H}_5\text{OH} \)) and explain its significance.
▶️Answer/Explanation
Ethanol contains the OH group (hydroxyl functional group).
This functional group gives ethanol its characteristic reactions, such as combustion, oxidation to ethanoic acid, and reaction with sodium to produce hydrogen gas.
All alcohols share this OH group, which is why they have similar chemical properties.
Structural Formula
A structural formula is an unambiguous description of how atoms in a molecule are arranged and bonded to each other. Unlike the molecular formula, which only shows the number of each atom, the structural formula shows the actual connectivity between atoms.
Key ideas:
- It makes clear how atoms are linked in a molecule
- It prevents ambiguity when different compounds can have the same molecular formula
- It is essential for understanding chemical behaviour and distinguishing isomers
Examples of structural formulae:
- Ethene (\( \text{C}_2\text{H}_4 \)) → \( \text{CH}_2 = \text{CH}_2 \)

- Methyl ethanoate (\( \text{C}_3\text{H}_6\text{O}_2 \)) → \( \text{CH}_3\text{COOCH}_3 \)
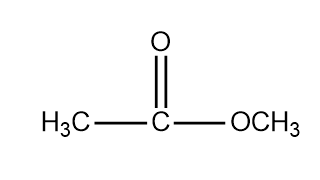
Example
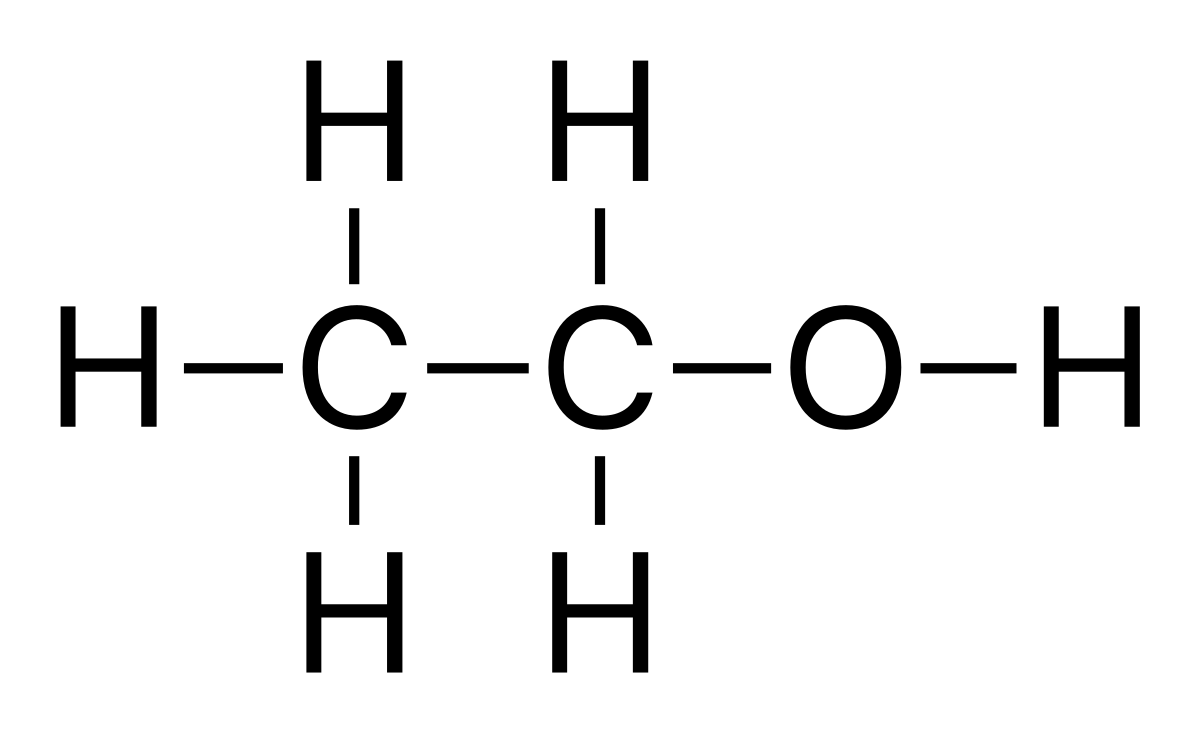
Explain why the structural formula is more useful than the molecular formula for ethanol.
▶️Answer/Explanation
The molecular formula of ethanol is \( \text{C}_2\text{H}_6\text{O} \).
However, this same formula could also represent dimethyl ether.
The structural formula \( \text{CH}_3\text{CH}_2\text{OH} \) clearly shows the hydroxyl group (OH) bonded to a carbon atom, distinguishing ethanol from dimethyl ether (\( \text{CH}_3\text{OCH}_3 \)). Thus, the structural formula removes ambiguity.
Structural Isomers
- Structural isomers are compounds that have the same molecular formula but different structural formulae.
- This means the number and type of atoms are identical, but the way these atoms are connected differs.
- As a result, isomers often show different physical properties (such as boiling points and melting points) and sometimes different chemical reactivity.
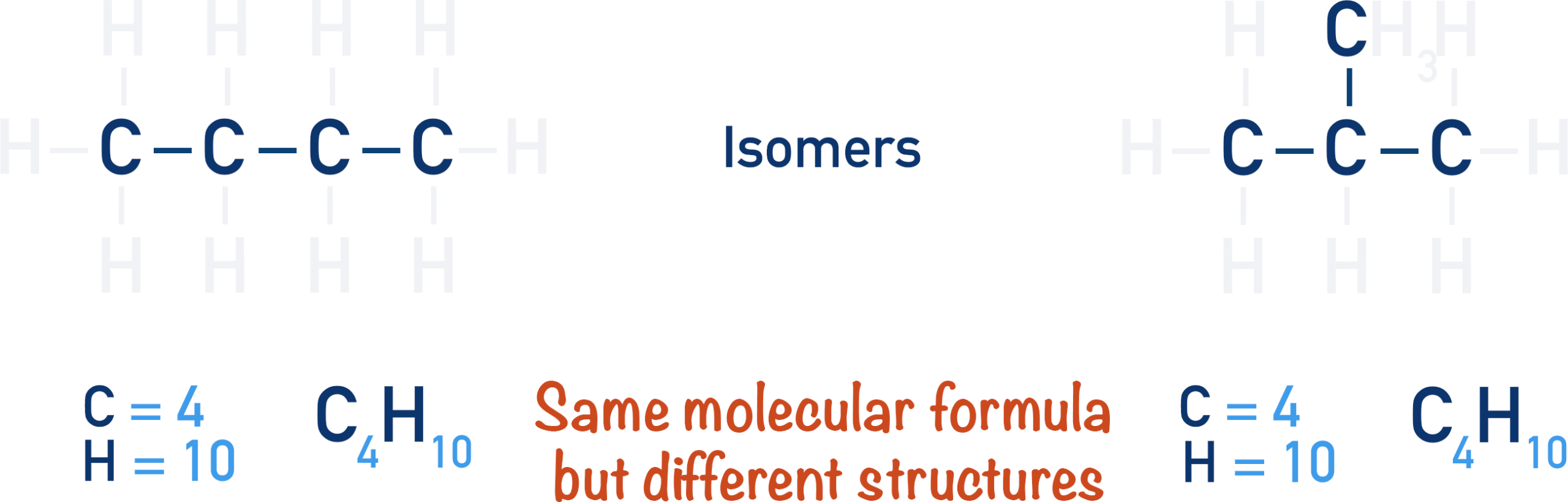
Key ideas:
- Same molecular formula
- Different arrangement of atoms
- Different structural formulae
- May show different properties even though they contain the same number and type of atoms
Examples of structural isomerism:
- Butene (\( \text{C}_4\text{H}_8 \)) →
\( \text{CH}_3\text{CH}_2\text{CH}=\text{CH}_2 \) (but-1-ene)
\( \text{CH}_3\text{CH}=\text{CHCH}_3 \) (but-2-ene)
Example
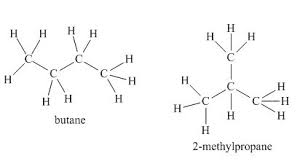
Explain why butane and 2-methylpropane are structural isomers.
▶️Answer/Explanation
Both butane and 2-methylpropane have the molecular formula \( \text{C}_4\text{H}_{10} \).
However, in butane the four carbon atoms form a straight chain, while in 2-methylpropane three carbon atoms form a chain with a branch (methyl group) attached to the middle carbon.
Their different connectivity means they are structural isomers.
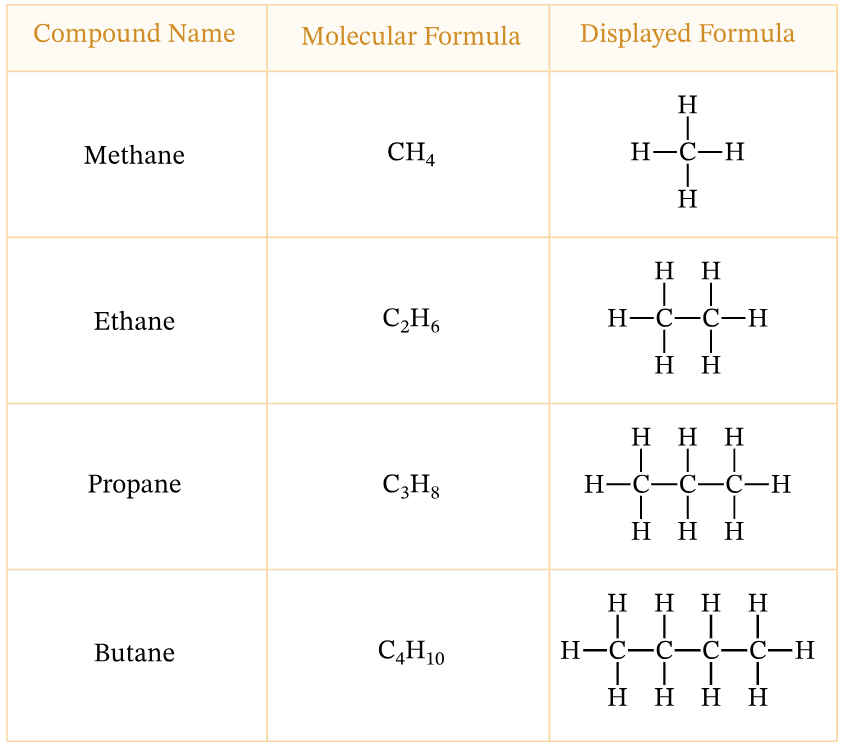
Homologous Series
Homologous Series
- A homologous series is a group of organic compounds arranged in a systematic way based on their structural and chemical similarities.
- All compounds in the same homologous series share the same functional group, which is the reactive part of the molecule and is responsible for their characteristic chemical properties.
- The members differ from one another in a regular and predictable manner, usually by a single \( \text{CH}_2 \) unit in the carbon chain. This structural relationship allows their general formula to be expressed in a simple algebraic form, which can be used to determine the molecular formula of any compound in the series.
- The gradual increase in the number of carbon atoms causes a steady change in physical properties such as boiling point, melting point, and density, while their chemical behavior remains broadly similar due to the identical functional group.
Example
Explain why alkanes are considered a homologous series.
▶️Answer/Explanation
Alkanes follow the general formula \( \text{C}_n\text{H}_{2n+2} \). Each member contains only carbon-carbon single bonds (C–C) and carbon-hydrogen bonds (C–H), so they share the same functional group and therefore similar chemical properties, such as combustion. Consecutive members differ by a \( \text{CH}_2 \) unit (methane → ethane → propane → butane, etc.), and as the chain length increases, physical properties like boiling points also increase in a gradual manner. These features make alkanes a clear example of a homologous series.
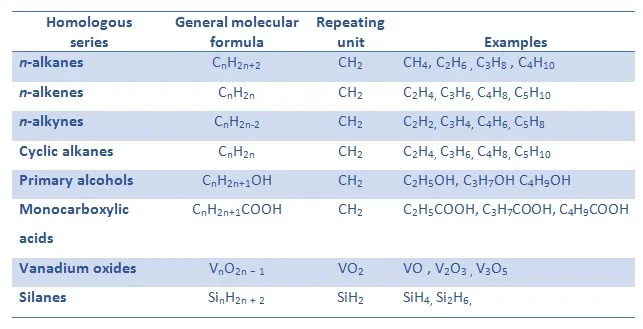
General Formulae of Compounds in a Homologous Series
- The general formula of a homologous series is an algebraic expression that represents the composition of all its members.
- It highlights the relationship between the number of carbon and hydrogen atoms in the molecules.
- Since every series has its own general formula, it can be used to deduce the molecular formula of any compound within that series, ensuring systematic study and classification of organic compounds.
General formulae of important series:
- Alkanes → \( \text{C}_n\text{H}_{2n+2} \)
- Alkenes → \( \text{C}_n\text{H}_{2n} \)
- Alcohols → \( \text{C}_n\text{H}_{2n+1}\text{OH} \)
- Carboxylic acids → \( \text{C}_n\text{H}_{2n+1}\text{COOH} \)
Example
Use the general formula of alkanes to deduce the molecular formula of pentane.
▶️Answer/Explanation
For alkanes, the general formula is \( \text{C}_n\text{H}_{2n+2} \).
In pentane, \( n = 5 \).
Substituting into the formula gives:
\( \text{C}_5\text{H}_{(2 \times 5 + 2)} = \text{C}_5\text{H}_{12} \).
Thus, the molecular formula of pentane is \( \text{C}_5\text{H}_{12} \).
General Characteristics of a Homologous Series
A homologous series displays a set of defining characteristics that account for both the similarities in chemical behavior and the systematic variation in physical properties. These characteristics are essential in identifying and classifying organic compounds into meaningful groups.
- All members contain the same functional group, which determines their similar chemical reactions.
- They obey the same general formula (for example, alkanes \( \text{C}_n\text{H}_{2n+2} \), alkenes \( \text{C}_n\text{H}_{2n} \)).
- Each successive member differs by a \( \text{CH}_2 \) unit, which leads to predictable changes in molecular mass and size.
- Physical properties such as boiling point, melting point, and density change gradually and systematically along the series as the chain length increases.
- Chemical properties remain similar across the series because the same functional group governs their reactivity.
Example
Describe how alcohols show the general characteristics of a homologous series.
▶️Answer/Explanation
All alcohols contain the hydroxyl group (–OH), which is the functional group responsible for their similar chemical reactions, such as reacting with sodium to release hydrogen gas. Their general formula is \( \text{C}_n\text{H}_{2n+1}\text{OH} \), which allows prediction of the composition of any alcohol. Each successive alcohol differs by a \( \text{CH}_2 \) unit (methanol → ethanol → propanol → butanol), and this leads to systematic changes in physical properties, for example, increasing boiling point with chain length. Despite these gradual physical changes, they undergo comparable chemical reactions because of the same –OH functional group. This demonstrates the general features of a homologous series.