Group I properties- CIE iGCSE Chemistry Notes - New Syllabus
Group I properties for iGCSE Chemistry Notes
Core Syllabus
- Describe the Group I alkali metals, lithium, sodium and potassium, as relatively soft metals with general trends down the group, limited to:
(a) decreasing melting point
(b) increasing density
(c) increasing reactivity - Predict the properties of other elements in Group I, given information about the elements
Group I alkali metals (Li, Na, K) and general trends
Group I alkali metals (Li, Na, K) and general trends
- Group 1 elements are known as alkali metals.
- They are all metals that are relatively soft and can be cut with a knife.
- They have low melting points compared to most other metals, and their melting points decrease down the group.
- Density generally increases down the group (except for an anomaly: potassium is less dense than sodium).
- They are highly reactive metals, and reactivity increases down the group.
Explanation of trends
- Melting point: decreases down the group because metallic bonding becomes weaker. The single outer electron is further from the nucleus → weaker attraction between cations and delocalised electrons.
- Density: increases down the group due to increasing atomic mass, although potassium is less dense than sodium because of its larger atomic volume.
- Reactivity: increases down the group because the outer electron is further from the nucleus → more easily lost → reacts more vigorously with water and halogens.
Example
Compare the melting points of lithium, sodium, and potassium and explain the trend.
▶️Answer/Explanation
Melting points decrease down the group:
Li (180°C) > Na (98°C) > K (63°C).
The outer electron is further from the nucleus → metallic bonding is weaker → lower melting point.
Example
Explain why potassium is more reactive than lithium.
▶️Answer/Explanation
Potassium has its single outer electron further from the nucleus compared to lithium → weaker attraction → electron lost more easily → reacts more vigorously with water and halogens.
Example
Predict the relative densities of Li, Na, and K and explain any anomalies.
▶️Answer/Explanation
Density trend: Li < Na < K (except K is slightly less dense than Na).
Density increases down the group due to increasing mass, but K has larger atomic volume → slightly lower density than expected.
The properties of other elements in Group 1
The properties of other elements in Group 1
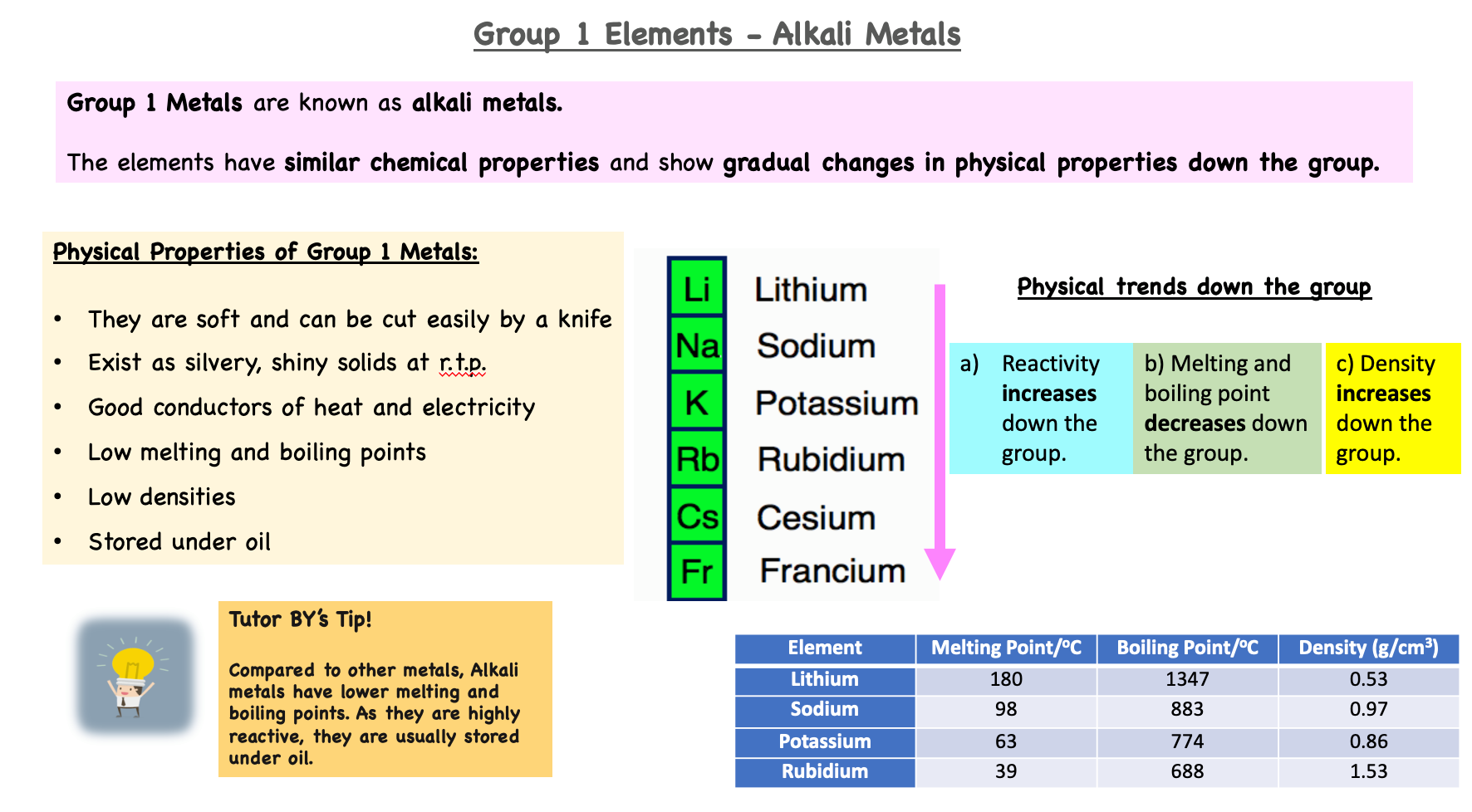
Properties of alkali metals can be predicted based on the trends observed in lithium, sodium, and potassium.
As you move down the group (Li → Na → K → Rb → Cs → Fr):
1. Melting and boiling points: decrease further down the group because metallic bonding weakens as the outer electron is further from the nucleus.
2. Density: generally increases due to higher atomic mass, but anomalies may occur due to increasing atomic volume.
3. Reactivity: increases down the group because the outer electron is more easily lost.
4. Appearance: all are soft, shiny metals that tarnish quickly in air.
5. Reaction with water: more vigorous down the group, producing hydroxides and hydrogen gas: \\( 2M + 2H_2O \\rightarrow 2MOH + H_2 \\)
6. Reaction with halogens: all form ionic halides: \\( 2M + X_2 \\rightarrow 2MX \\)
Example
Predict the reactivity of cesium with water compared to potassium.
▶️Answer/Explanation
Cesium is further down Group 1 than potassium → outer electron is further from nucleus → more easily lost → reacts more violently with water.
Example
Predict the melting point of rubidium relative to lithium and sodium.
▶️Answer/Explanation
Melting point decreases down the group: Li > Na > Rb.
Rubidium has weaker metallic bonding because its outer electron is further from the nucleus → lower melting point.
Example
Predict the density trend for rubidium and cesium.
▶️Answer/Explanation
Density generally increases down the group due to higher atomic mass, although large atomic volume may slightly reduce the expected density. Rubidium < cesium.
