Metallic bonding- CIE iGCSE Chemistry Notes - New Syllabus
Metallic bonding for iGCSE Chemistry Notes
Core Syllabus
- Describe metallic bonding as the electrostatic attraction between the positive ions in a giant metallic lattice and a ‘sea’ of delocalised electrons
- Explain in terms of structure and bonding the properties of metals:
(a) good electrical conductivity
(b) malleability and ductility
Metallic Bonding
Metallic Bonding
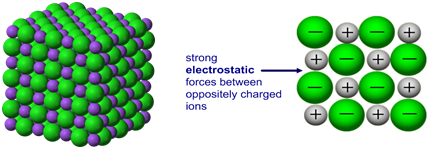
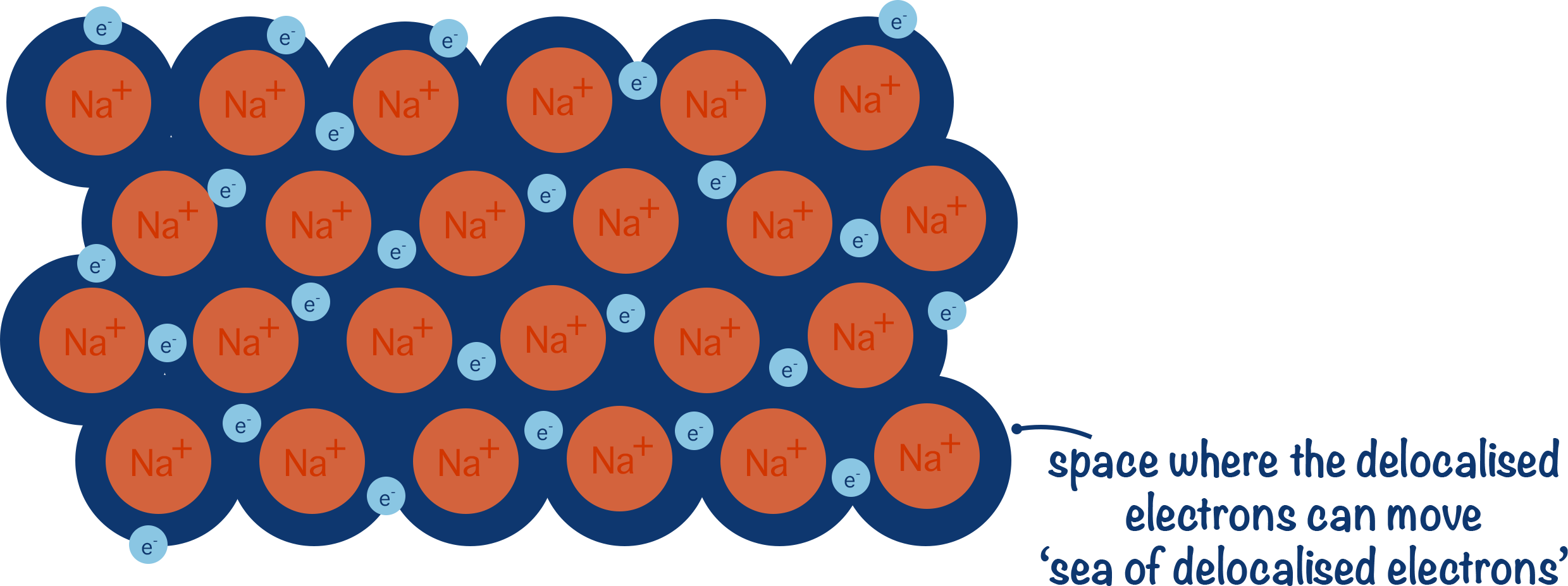
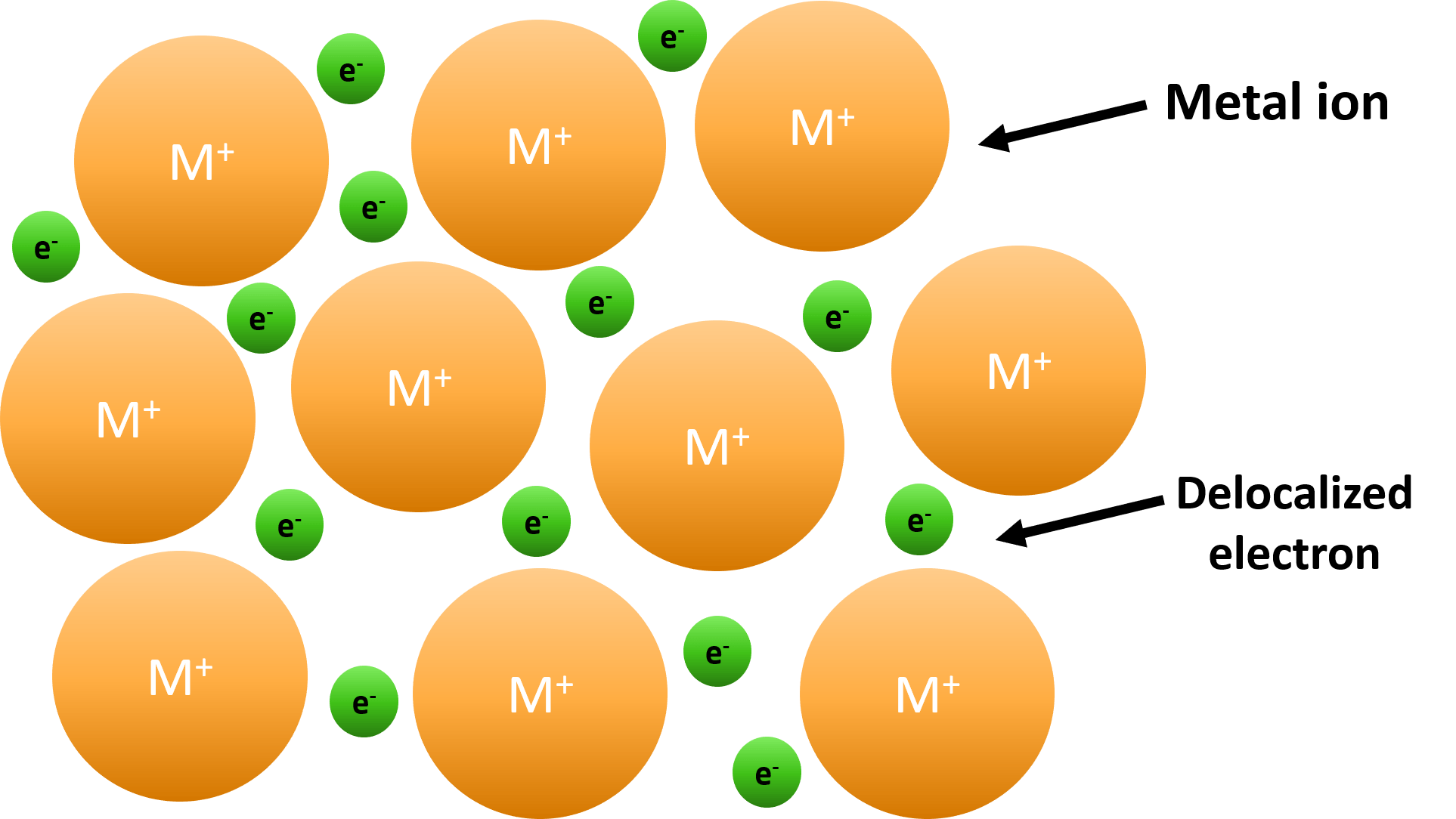
Metallic bonding is the strong electrostatic attraction between positive metal ions arranged in a regular lattice and a surrounding ‘sea’ of delocalized electrons.
How it forms:
- Metal atoms lose their outer shell electrons to become positive ions (cations).
- The lost electrons are delocalized, meaning they are free to move throughout the structure.
- The metal is held together by the attraction between these free-moving negative electrons and the fixed positive metal ions.
Structure:
- Metals consist of a giant metallic lattice – a regular 3D arrangement of positive metal ions.
- The delocalized electrons move freely between the ions and act like a “glue” that holds the entire structure together.
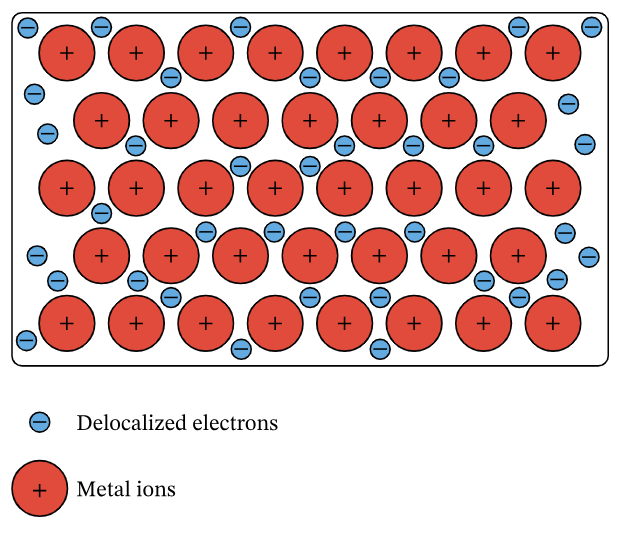
Diagram Description:
- Draw regularly spaced circles (metal ions) with ‘+’ signs inside them.
- Surround them with small dots or ‘e⁻’ symbols to represent delocalized electrons.
- Label: positive ions in fixed positions and delocalized electrons moving freely.
Example
Describe metallic bonding in sodium metal using particle types and forces involved.
▶️Answer/Explanation
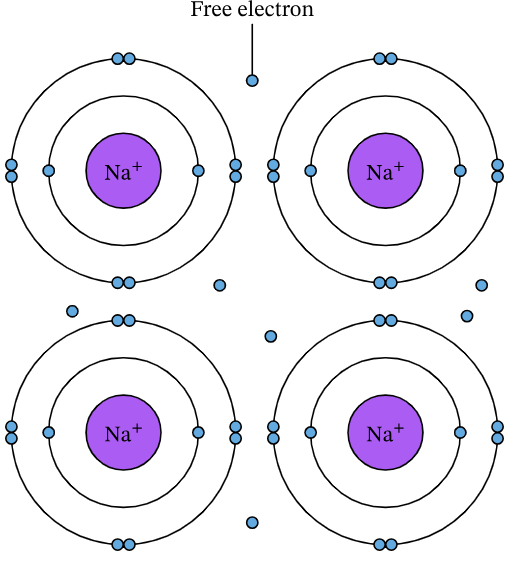
Sodium is a metal and consists of a giant metallic lattice. Each sodium atom loses one electron to become a Na+ ion. These electrons become delocalized and form a ‘sea’ of free-moving electrons that surround the metal ions.
The metallic bond is the electrostatic attraction between the Na+ ions and the delocalized electrons. This bonding gives sodium its metallic properties such as conductivity and malleability.
Example
Explain the metallic bonding in iron and how it contributes to its physical properties.
▶️Answer/Explanation
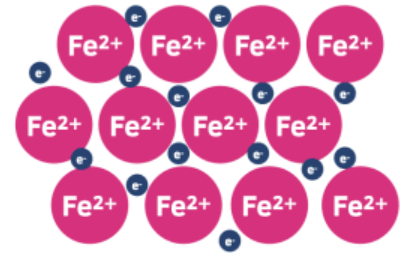
Iron forms a giant metallic lattice structure where each iron atom loses electrons to form positive Fe2+ or Fe3+ ions. The lost electrons become delocalized and move freely throughout the structure.
Metallic bonding is the electrostatic attraction between these positive iron ions and the sea of delocalized electrons. This strong attraction holds the structure together and gives iron its characteristic metallic properties such as:
- High electrical conductivity – due to free electrons moving through the metal
- Malleability and ductility – layers of ions can slide over one another without breaking the bond
- High melting point – strong forces require a lot of energy to break
Properties of Metals
Properties of Metals
Metals have a giant metallic lattice structure. Positive metal ions are arranged in layers and surrounded by a sea of delocalized electrons. The strong electrostatic attraction between these particles is responsible for the characteristic properties of metals.
| Property | Explanation in terms of structure and bonding |
|---|---|
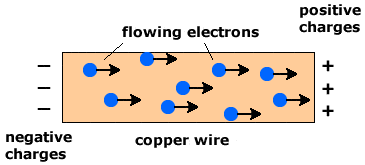
Good electrical conductivity | Metals conduct electricity because of the presence of delocalized electrons that move freely throughout the structure. When a potential difference is applied, these electrons drift in one direction, allowing an electric current to pass through the metal. Unlike ionic compounds, metals conduct electricity in both solid and molten states because these electrons are always free to move. |
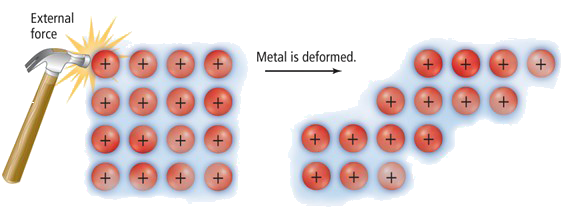
Malleability
| In a metallic lattice, layers of positive ions are arranged in a regular pattern. These layers can slide over each other when force is applied. Because the delocalized electrons move with the layers and continue to hold the structure together, the metal does not break. This allows metals to be bent or shaped without cracking. |
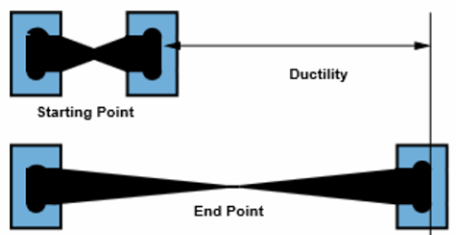
Ductility
| Like malleability, ductility arises because the layers of metal ions can shift without breaking the metallic bond. The flexible structure and the presence of delocalized electrons mean the metal can be stretched into long, thin wires without snapping. |
High melting and boiling points
| Metallic bonding is very strong because of the strong electrostatic forces between the closely packed positive ions and the negatively charged sea of electrons. A large amount of thermal energy is required to overcome these forces, so metals have high melting and boiling points. This makes them useful for applications involving heat or high temperatures. |
| High density | Most metals have tightly packed ions arranged in regular layers, which means there are many atoms in a small volume. This makes metals very dense compared to other types of substances (like non-metals or molecular compounds). |
| Sonorous | Metals produce a ringing sound when struck. This is because the metal lattice structure allows sound waves to travel efficiently through the tightly bonded particles. |
| Lustrous (shiny) | Metals are shiny because the delocalized electrons reflect light off the surface of the metal. This is why freshly cut or polished metals appear bright and lustrous. |
Example
Why can copper conduct electricity in both solid and molten states?
▶️Answer/Explanation
Copper is a metal, so it has a giant metallic lattice made up of positive Cu2+ ions and a sea of delocalized electrons. These electrons are free to move through the structure.
Because the electrons are mobile even in the solid state, copper can conduct electricity without needing to be molten. When a voltage is applied, these electrons drift and create an electric current.
Example
Explain why metals like aluminium and gold are malleable.
▶️Answer/Explanation
Metals have a layered lattice of positive ions held together by a sea of delocalized electrons.
When a force is applied, these layers can slide over one another without breaking the strong metallic bonds because the electrons move with the ions and continue to hold the structure together. This allows the metal to be bent or shaped without cracking.