Naming organic compounds- CIE iGCSE Chemistry Notes - New Syllabus
Naming organic compounds for iGCSE Chemistry Notes
Core Syllabus
- Name and draw the displayed formulae of:
(a) methane and ethane
(b) ethene
(c) ethanol
(d) ethanoic acid
(e) the products of the reactions stated in sections 11.4–11.7 - State the type of compound present, given a chemical name ending in -ane, -ene, -ol, or -oic acid or from a molecular formula or displayed formula
Supplement Syllabus
- Name and draw the structural and displayed formulae of unbranched:
(a) alkanes
(b) alkenes, including but-1-ene and but-2-ene
(c) alcohols, including propan-1-ol, propan-2-ol, butan-1-ol and butan-2-ol
(d) carboxylic acids containing up to four carbon atoms per molecule - Name and draw the displayed formulae of the unbranched esters which can be made from unbranched alcohols and carboxylic acids, each containing up to four carbon atoms
Name and draw the displayed formulae
Name and draw the displayed formulae
This section focuses on naming simple organic compounds and representing them with displayed formulae. Displayed formulae show all atoms and the bonds between them, helping to identify the functional group and understand chemical reactivity.
(a) Methane and Ethane
- Methane is the simplest alkane containing one carbon atom bonded to four hydrogen atoms.
- Ethane is the next member in the alkane series with two carbons connected by a single bond, each carbon also bonded to hydrogens.
- These compounds are saturated hydrocarbons because they contain only single covalent bonds.
Example
Draw the displayed formulae of methane and ethane.
▶️Answer/Explanation
Methane: \( \text{CH}_4 \)
Displayed formula:
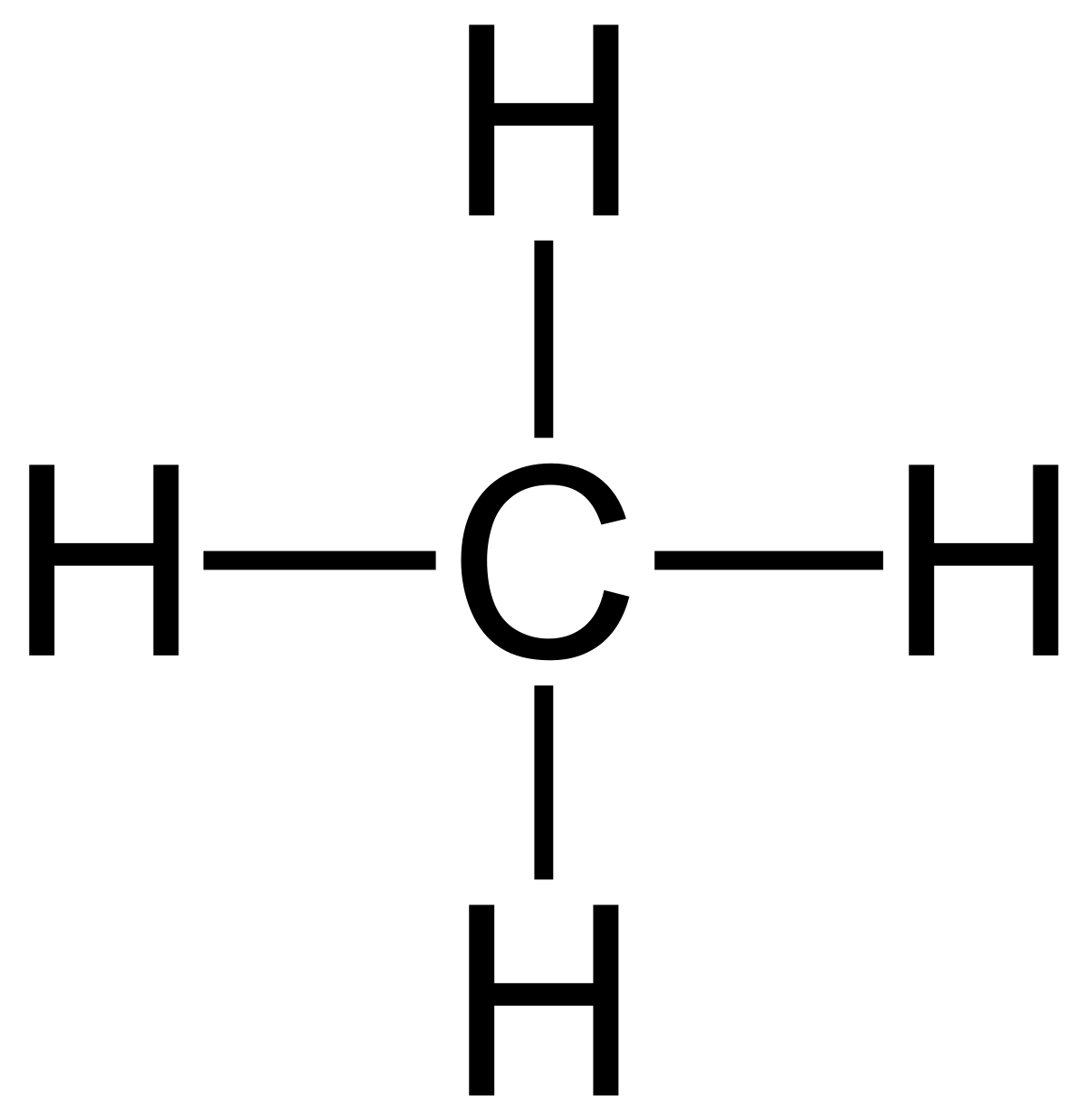
Explanation: Each hydrogen forms a single covalent bond with carbon. The tetrahedral shape is implied.
Ethane: \( \text{C}_2\text{H}_6 \)
Displayed formula: H–C–C–H with three hydrogens attached to each carbon:
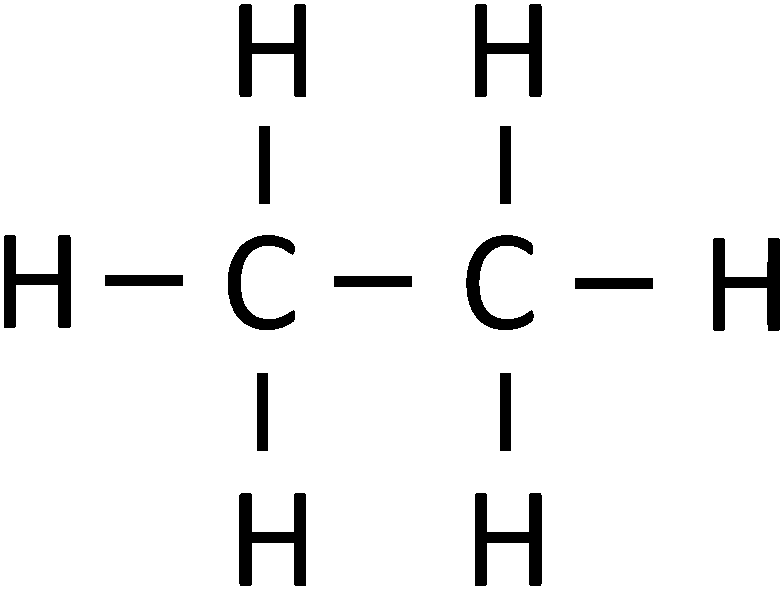
Explanation: Both carbons have four single bonds, satisfying the octet rule. This confirms ethane is a saturated hydrocarbon.
(b) Ethene
- Ethene is an alkene with a C=C double bond, making it unsaturated.
- The double bond consists of one sigma and one pi bond, which is more reactive than a single bond.
- This is the simplest unsaturated hydrocarbon, and the functional group is the double bond.
Example
Draw the displayed formula of ethene.
▶️Answer/Explanation
Ethene: \( \text{C}_2\text{H}_4 \)
Displayed formula: H–C=C–H with two hydrogens on each carbon:
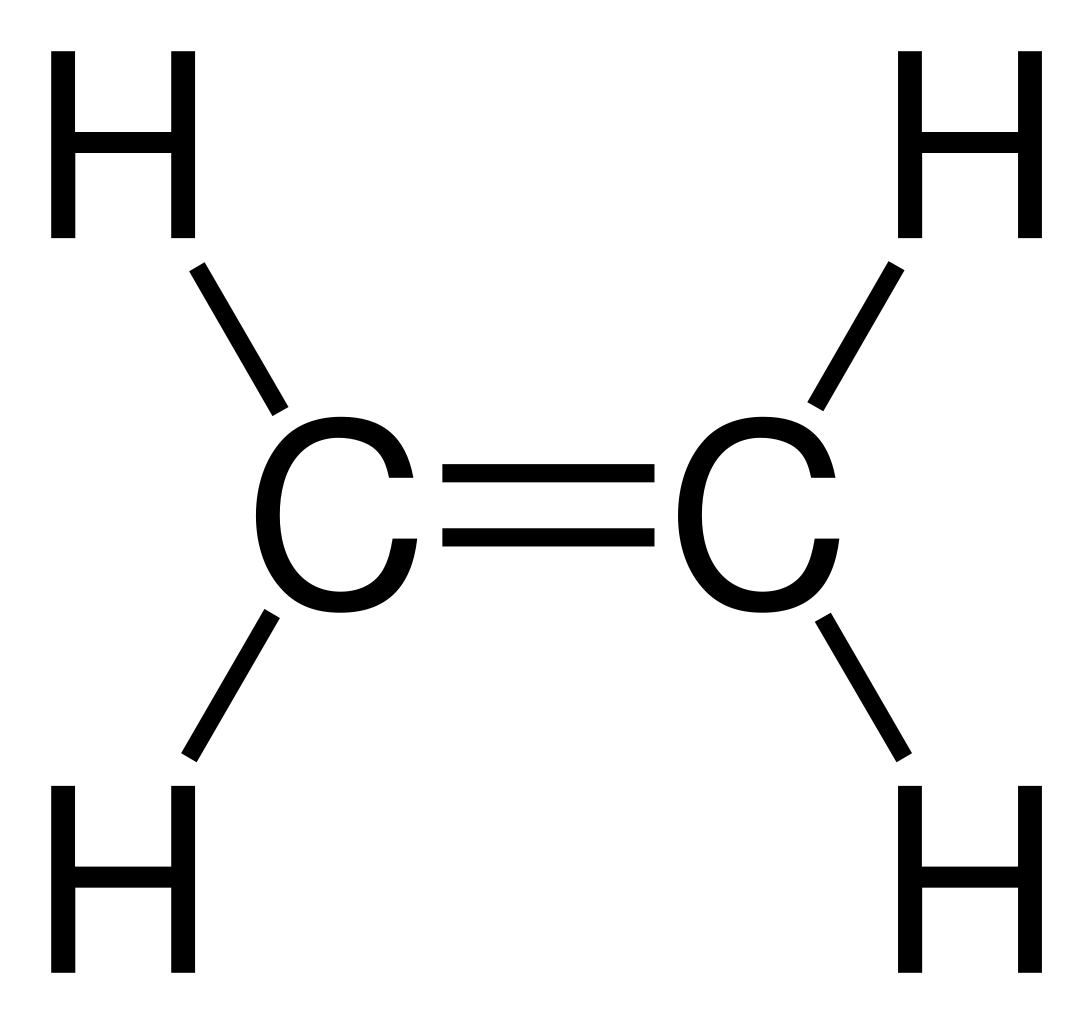
Explanation: The double bond introduces unsaturation and higher reactivity compared to alkanes, important for addition reactions.
(c) Ethanol
- Ethanol is an alcohol, containing the –OH functional group.
- The hydroxyl group is polar, enabling hydrogen bonding, which affects boiling point and solubility in water.
- It is important to distinguish alcohols from alkanes and alkenes by identifying this functional group in the displayed formula.
Example
Draw the displayed formula of ethanol.
▶️Answer/Explanation
Ethanol: \( \text{C}_2\text{H}_5\text{OH} \)
Displayed formula:
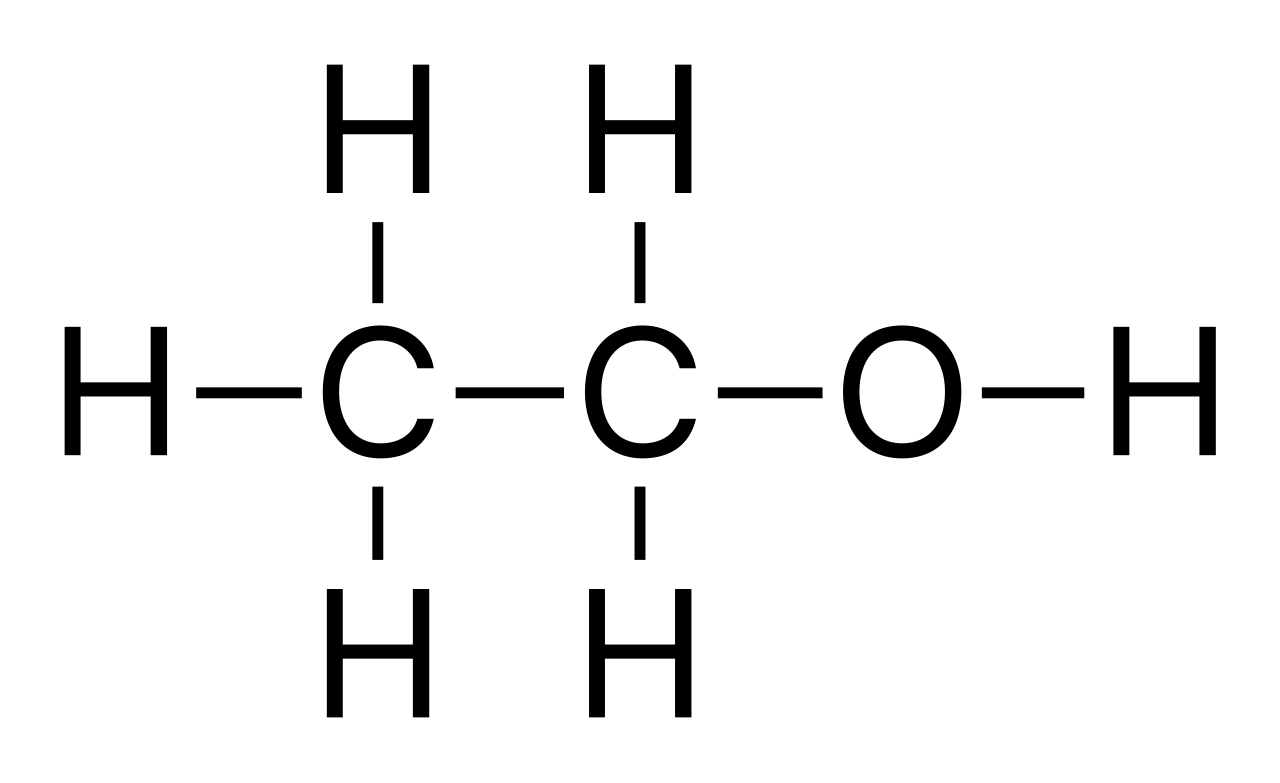
Explanation: The –OH group is the functional group. Ethanol is polar, can form hydrogen bonds, and participates in characteristic alcohol reactions (like combustion and esterification).
(d) Ethanoic Acid
- Ethanoic acid is a carboxylic acid with the –COOH functional group.
- It is weakly acidic and reacts with metals, bases, and carbonates.
- Identifying the carboxyl group in the displayed formula is essential to predict chemical reactions.
Example
Draw the displayed formula of ethanoic acid.
▶️Answer/Explanation
Ethanoic acid: \( \text{CH}_3\text{COOH} \)
Displayed formula:
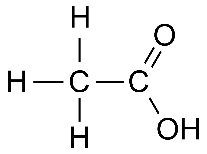
Explanation: The carbonyl (C=O) and hydroxyl (–OH) groups together form –COOH. This functional group gives acids their characteristic reactions and properties.
(e) Products of reactions
This point focuses on understanding the products formed when different classes of organic compounds react, based on the reactions covered in alkanes, alkenes, alcohols, and carboxylic acids:
Example
Methane reacts with chlorine. Draw the formula of compound formed.
▶️Answer/Explanation
Reaction:
\( \text{CH}_4 + \text{Cl}_2 \xrightarrow{\text{UV}} \text{CH}_3\text{Cl} + \text{HCl} \)
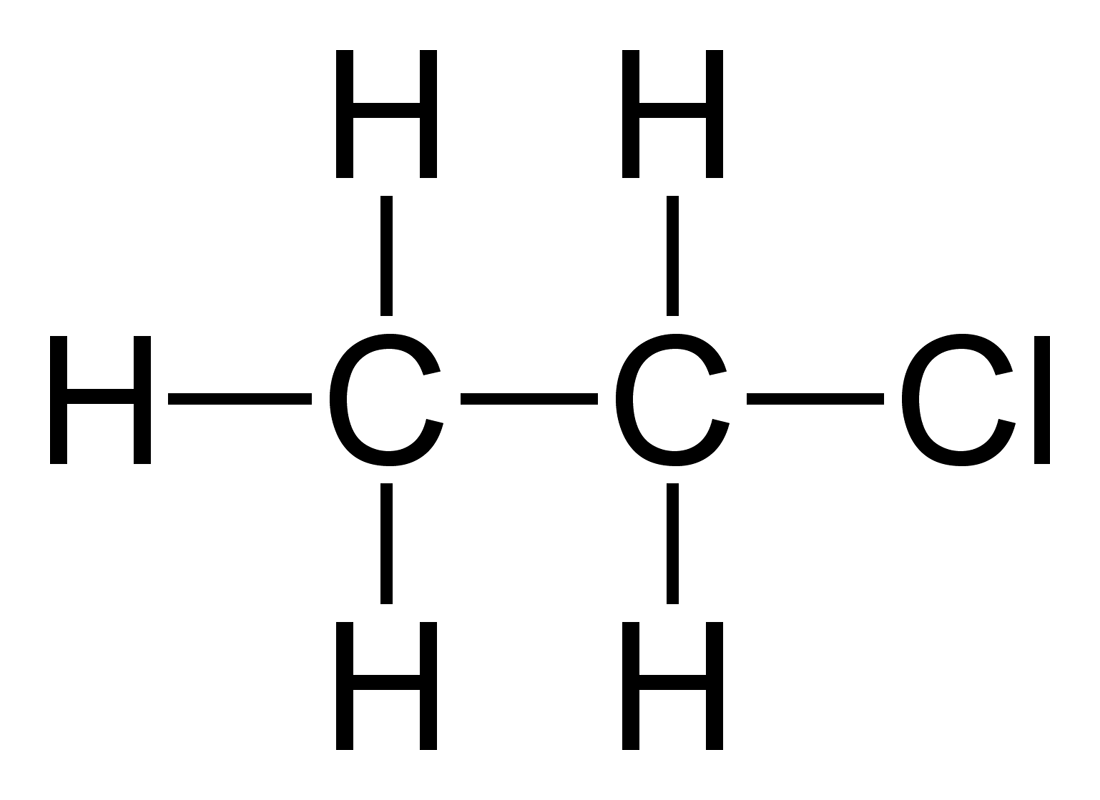
Explanation: Alkanes are generally unreactive due to strong C–H and C–C single bonds. In the presence of UV light, one hydrogen atom in methane is replaced by a chlorine atom in a substitution reaction. Chloromethane (CH₃Cl) is formed along with HCl. This illustrates how alkanes undergo substitution rather than addition.
Example
Ethene reacts with bromine. Draw the formula of compound formed.
▶️Answer/Explanation
Reaction:
\( \text{CH}_2=CH_2 + \text{Br}_2 \rightarrow \text{CH}_2\text{Br}-\text{CH}_2\text{Br} \)
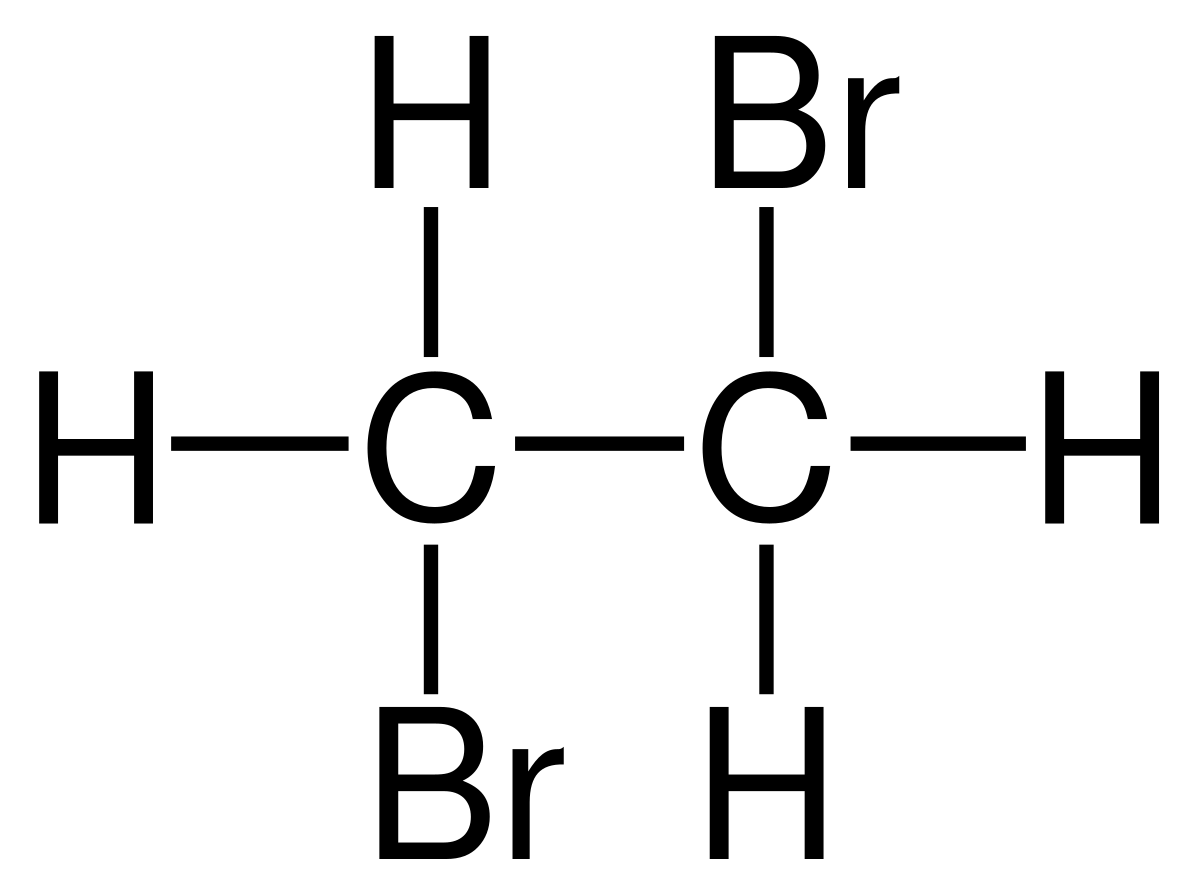
Explanation: Alkenes contain a C=C double bond which is electron-rich and reactive. Bromine adds across the double bond in an addition reaction, forming a dibromo compound. This demonstrates that alkenes are more reactive than alkanes because of the unsaturation.
Example
Ethanol is burned. Draw the formula of compound formed.
▶️Answer/Explanation
Reaction:
\( \text{C}_2\text{H}_5\text{OH} + 3\text{O}_2 \rightarrow 2\text{CO}_2 + 3\text{H}_2\text{O} \)
![]()
Explanation: Alcohols like ethanol burn in oxygen to produce carbon dioxide and water. The –OH group does not change during combustion; it affects polarity and solubility but not the energy released. This shows that alcohols are reactive in combustion but stable otherwise.
Example
Ethanoic acid reacts with ethanol. Draw the formula of compound formed.
▶️Answer/Explanation
Reaction:
\( \text{CH}_3\text{COOH} + \text{CH}_3\text{CH}_2\text{OH} \xrightarrow{\text{H}_2\text{SO}_4} \text{CH}_3\text{COOCH}_2\text{CH}_3 + \text{H}_2\text{O} \)
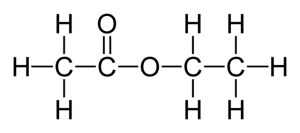
Explanation: Carboxylic acids react with alcohols in the presence of an acid catalyst to form esters and water. The –COOH group reacts with the –OH of the alcohol, forming a new functional group –COO–. This reaction shows how functional groups control the type of products formed in organic reactions.
Identifying the type of compound
Identifying the type of compound
This point focuses on identifying the type of organic compound given a chemical name, molecular formula, or displayed formula. Recognizing the functional group is key to predicting chemical properties and reactivity.
(a) Identifying from the chemical name ending
- Names ending in -ane indicate alkanes, which are saturated hydrocarbons with single \( \text{C–C} \) bonds only.
- Names ending in -ene indicate alkenes, which are unsaturated hydrocarbons containing at least one \( \text{C=C} \) double bond.
- Names ending in -ol indicate alcohols, which contain the hydroxyl functional group \( \text{–OH} \).
- Names ending in -oic acid indicate carboxylic acids, which contain the carboxyl group \( \text{–COOH} \).
(b) Identifying from molecular formula
By analyzing the ratio of carbon to hydrogen and the presence of oxygen, we can determine the compound type:
- Molecular formula \( \text{C}_n\text{H}_{2n+2} \) → Alkane
- Molecular formula \( \text{C}_n\text{H}_{2n} \) → Alkene
- Molecular formula \( \text{C}_n\text{H}_{2n+1}\text{OH} \) → Alcohol
- Molecular formula \( \text{C}_n\text{H}_{2n+1}\text{COOH} \) → Carboxylic acid
(c) Identifying from displayed formula
The displayed formula shows all atoms and bonds. Identification is done by locating the functional group:
- Only \( \text{C–C} \) single bonds → Alkane
- Contains \( \text{C=C} \) double bond → Alkene
- Contains \( \text{–OH} \) group → Alcohol
- Contains \( \text{–COOH} \) group → Carboxylic acid
Example
A compound has the molecular formula \( \text{C}_2\text{H}_4 \). Identify the type of compound using the chemical name, molecular formula, and displayed formula.
▶️Answer/Explanation
From the chemical name: The compound is called ethene. The name ending -ene shows it is an alkene.
From the molecular formula: The formula fits the pattern \( \text{C}_n\text{H}_{2n} \), which corresponds to alkenes.
From the displayed formula: The structure \( \text{H}_2\text{C} = \text{CH}_2 \) contains a carbon–carbon double bond, confirming it is an alkene.
Final Answer: The compound is ethene, an alkene.
Example
A compound has the molecular formula \( \text{C}_2\text{H}_4\text{O}_2 \). Identify the type of compound using the chemical name, molecular formula, and displayed formula.
▶️Answer/Explanation
From the chemical name: The compound is called ethanoic acid. The name ending -oic acid shows it is a carboxylic acid.
From the molecular formula: \( \text{C}_2\text{H}_4\text{O}_2 \) could represent either an alcohol with an extra oxygen or a carboxylic acid. The exact type cannot be confirmed from the formula alone.
From the displayed formula: The structure \( \text{CH}_3\text{–COOH} \) shows the carboxyl group \( \text{–COOH} \), confirming it is a carboxylic acid.
Final Answer: The compound is ethanoic acid, a carboxylic acid.
Naming and drawing structural and displayed formulae of unbranched compounds
Naming and drawing structural and displayed formulae of unbranched compounds
This point focuses on naming and representing unbranched alkanes, alkenes, alcohols, and carboxylic acids containing up to four carbon atoms. Correct naming is based on the IUPAC rules, and drawing structural and displayed formulae helps visualize the molecules.
(a) Alkanes
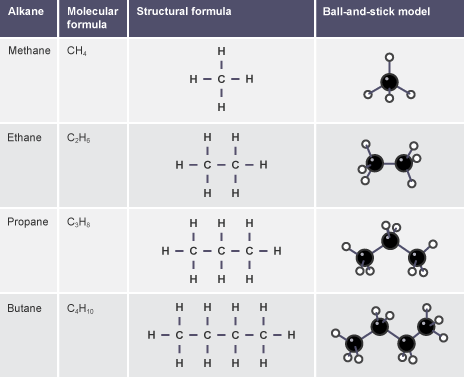
- Methane → \( \text{CH}_4 \) → single carbon, all single bonds
- Ethane → \( \text{C}_2\text{H}_6 \) → two carbons, all single bonds
- Propane → \( \text{C}_3\text{H}_8 \) → three carbons, all single bonds
- Butane → \( \text{C}_4\text{H}_{10} \) → four carbons, all single bonds
Structural formulae show the \( \text{C–C} \) backbone with H atoms arranged to satisfy valency. Displayed formulae show all bonds explicitly.
Example
Draw the structural and displayed formulae of butane:
▶️Answer/Explanation
Structural formula: \( \text{CH}_3–\text{CH}_2–\text{CH}_2–\text{CH}_3 \)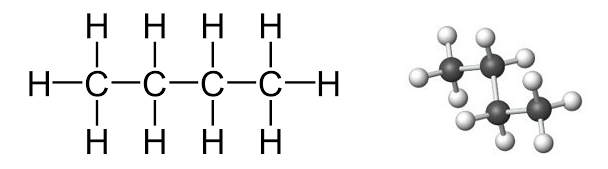
with H atoms attached to each C to complete four bonds
(b) Alkenes
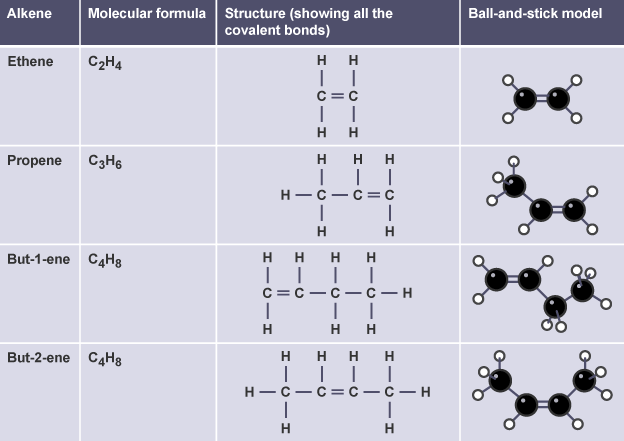
- Ethene → \( \text{C}_2\text{H}_4 \) → one double bond between two carbons
- Propene → \( \text{C}_3\text{H}_6 \) → double bond between first and second carbon
- But-1-ene → \( \text{C}_4\text{H}_8 \) → double bond at first carbon
- But-2-ene → \( \text{C}_4\text{H}_8 \) → double bond at second carbon
Structural and displayed formulae explicitly show the position of the double bond.
Example
Draw but-2-ene structural formula:
▶️Answer/Explanation
\( \text{CH}_3–\text{CH}=\text{CH}–\text{CH}_3 \)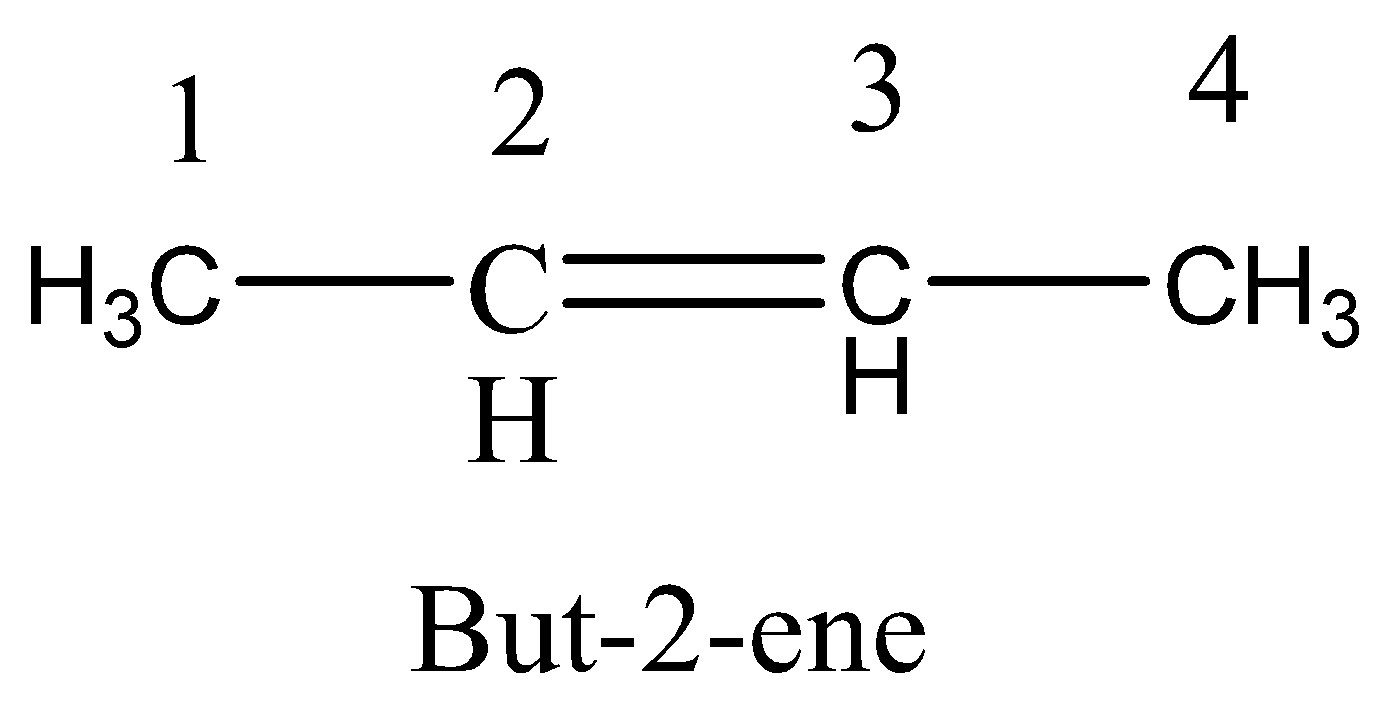
(c) Alcohols
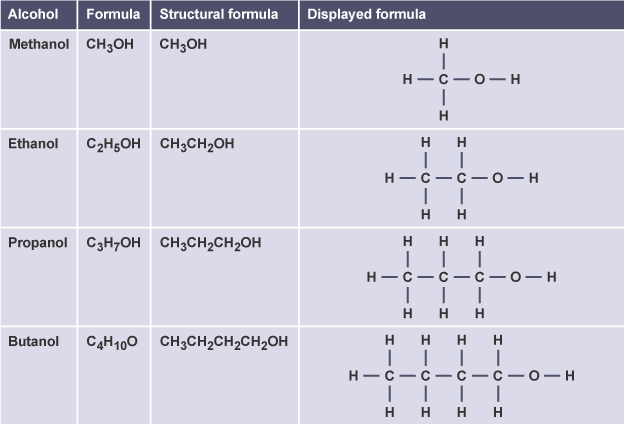
- Methanol → \( \text{CH}_3\text{OH} \) → hydroxyl on first carbon
- Ethanol → \( \text{C}_2\text{H}_5\text{OH} \) → hydroxyl on first carbon
- Propan-1-ol → \( \text{CH}_3\text{CH}_2\text{CH}_2\text{OH} \) → \( \text{–OH} \) on first carbon
- Propan-2-ol → \( \text{CH}_3\text{CHOH}\text{CH}_3 \) → \( \text{–OH} \) on second carbon
- Butan-1-ol → \( \text{CH}_3\text{CH}_2\text{CH}_2\text{CH}_2\text{OH} \) → \( \text{–OH} \) on first carbon
- Butan-2-ol → \( \text{CH}_3\text{CH}_2\text{CHOH}\text{CH}_3 \) → \( \text{–OH} \) on second carbon
Structural and displayed formulae show the \( \text{–OH} \) group attached to the correct carbon.
Example
Draw propan-2-ol displayed formula:
▶️Answer/Explanation
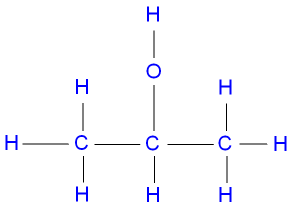
(d) Carboxylic acids
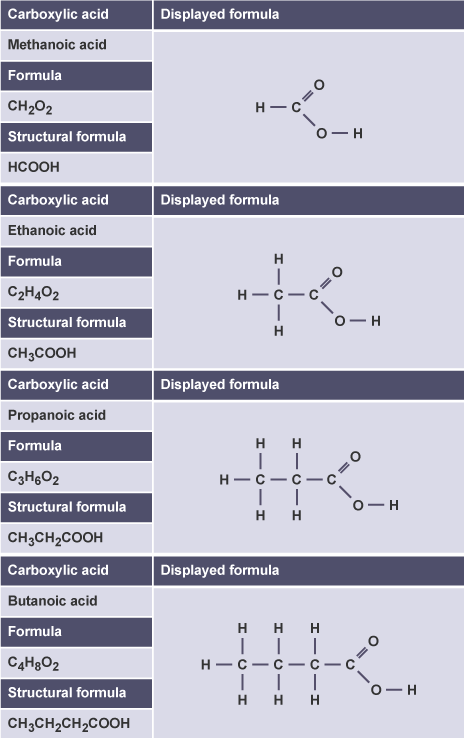
- Methanoic acid → \( \text{HCOOH} \) → one carbon
- Ethanoic acid → \( \text{CH}_3\text{COOH} \) → two carbons
- Propanoic acid → \( \text{CH}_3\text{CH}_2\text{COOH} \) → three carbons
- Butanoic acid → \( \text{CH}_3\text{CH}_2\text{CH}_2\text{COOH} \) → four carbons
Structural and displayed formulae show the \( \text{–COOH} \) group at the end of the carbon chain.
Example
Draw butanoic acid structural formula:
▶️Answer/Explanation
\( \text{CH}_3–\text{CH}_2–\text{CH}_2–\text{COOH} \)
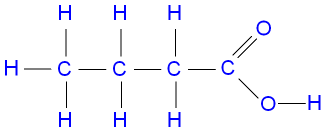
Displayed formula shows H atoms attached to C atoms and O–H bond in carboxyl group
Naming and drawing displayed formulae of unbranched esters
Naming and drawing displayed formulae of unbranched esters
This point focuses on understanding esters formed from unbranched alcohols and carboxylic acids, each containing up to four carbon atoms. Esters are formed via esterification, where a carboxylic acid reacts with an alcohol in the presence of an acid catalyst to produce an ester and water.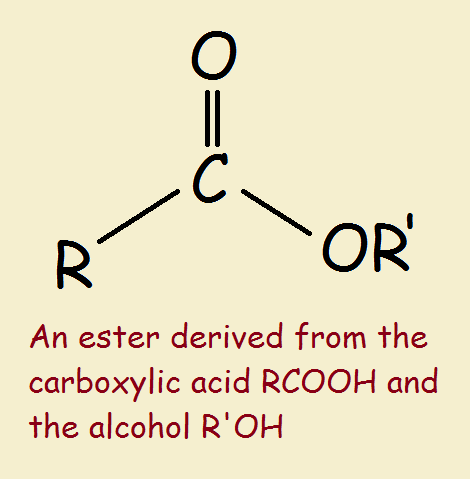
(a) General formula and naming of esters
- Esters have the general formula \( \text{R–COO–R’} \), where R is from the carboxylic acid and R’ is from the alcohol.
- The name of an ester is derived from the alcohol and acid: the alkyl part from the alcohol comes first, followed by the acid part with -oate ending.
(b) Esters from up to four carbon alcohols and acids
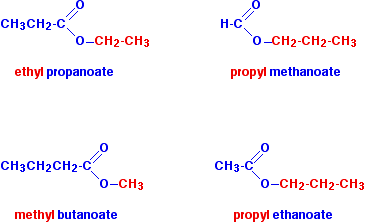
- Methyl methanoate → from methanol + methanoic acid
- Methyl ethanoate → from methanol + ethanoic acid
- Ethyl ethanoate → from ethanol + ethanoic acid
- Propyl ethanoate → from propanol + ethanoic acid
- Butyl methanoate → from butanol + methanoic acid
Example
Draw the structural and displayed formula of ethyl ethanoate:
▶️Answer/Explanation
Reaction: \( \text{CH}_3\text{COOH} + \text{C}_2\text{H}_5\text{OH} \rightarrow \text{CH}_3\text{COOCH}_2\text{CH}_3 + \text{H}_2\text{O} \)
Structural formula: \( \text{CH}_3\text{–COO–CH}_2\text{–CH}_3 \)
Displayed formula shows:
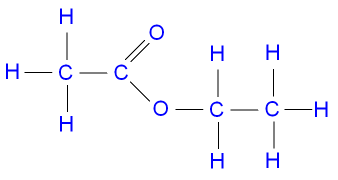
The –COO– group connects the acid and alcohol parts, showing all hydrogen atoms attached to carbon.
