Noble gases- CIE iGCSE Chemistry Notes - New Syllabus
Noble gases for iGCSE Chemistry Notes
Core Syllabus
- Describe the Group VIII noble gases as unreactive, monatomic gases and explain this in terms of electronic configuration
Noble Gases
Noble Gases
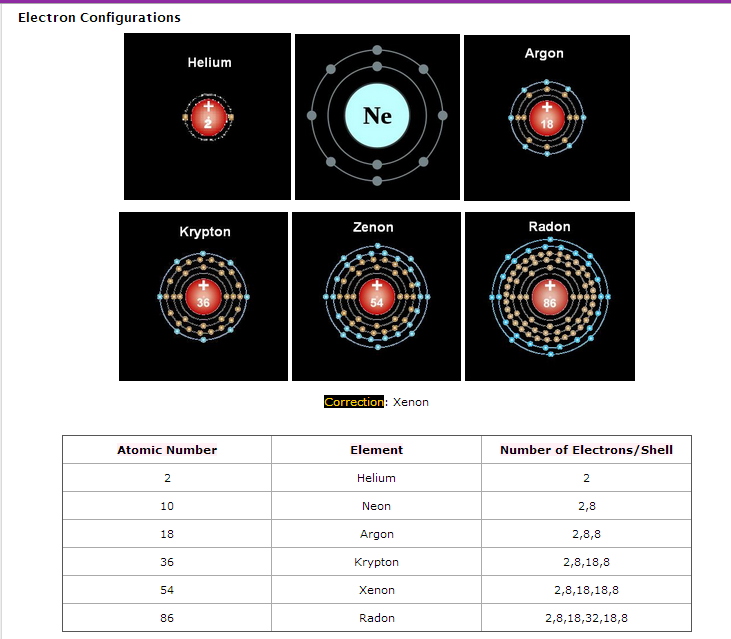
- Group VIII elements (He, Ne, Ar, Kr, Xe, Rn) are called noble gases.
- They are all monatomic gases at room temperature and extremely unreactive.
Electronic configuration and unreactivity
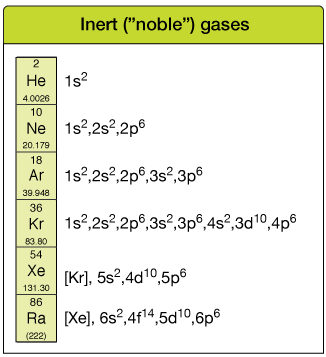
- Noble gases have full outer electron shells:
- Helium: 1s²
- Neon: 2s²2p⁶
- Argon: 3s²3p⁶
- Krypton: 4s²4p⁶
- Xenon: 5s²5p⁶
- Radon: 6s²6p⁶
- Full valence shells → atoms are stable → no tendency to gain, lose, or share electrons → chemically inert.
- Monatomic nature arises because there is no need to form bonds with other atoms; they exist independently as single atoms.
Physical properties
- All are colourless, tasteless, and odourless gases at room temperature.
- Extremely low chemical reactivity; most do not form compounds under normal conditions.
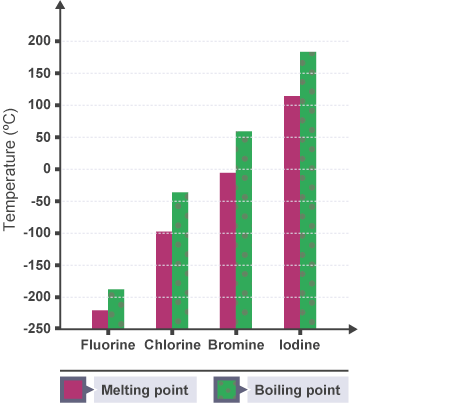
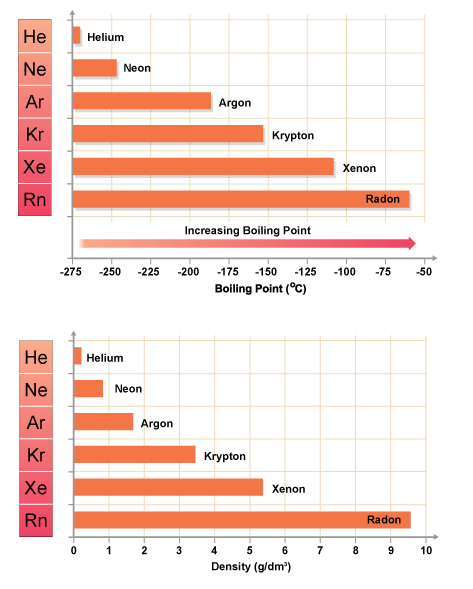
- Melting and boiling points increase down the group:
- Reason: van der Waals forces increase with atomic size and electron cloud size → more energy required to change state.
- Trend: He < Ne < Ar < Kr < Xe < Rn.
Chemical insights
- Noble gases rarely form compounds because of their full valence shells, but heavier gases (Xe, Kr) can form compounds with highly electronegative elements like fluorine and oxygen under extreme conditions.
- Examples of noble gas compounds: XeF₂, XeF₄, XeO₃.
- Use in inert atmospheres:
- Helium in gas-filled balloons and as a cooling medium.
- Argon in welding to prevent oxidation.
- Neon, krypton, xenon in lighting applications.
Example
Explain why neon is chemically inert.
▶️Answer/Explanation
Neon has a full outer shell (2s²2p⁶).
Full valence shell → stable configuration → no tendency to gain, lose, or share electrons → chemically unreactive.
Example
Explain why helium exists as a monatomic gas.
▶️Answer/Explanation
Helium has a full 1s² valence shell → extremely stable.
No bonding needed → exists as individual atoms (monatomic gas).
Example
Predict the trend in boiling points of noble gases down the group and explain.
▶️Answer/Explanation
Boiling points increase down the group: He < Ne < Ar < Kr < Xe < Rn.
Larger atoms → larger electron clouds → stronger van der Waals forces → more energy needed to overcome these forces.
Example
Explain why xenon can form compounds while helium does not.
▶️Answer/Explanation
Xenon has larger, more easily polarised electron cloud → valence electrons can be influenced by highly electronegative elements (like F, O) under extreme conditions → forms compounds such as XeF2.
Helium’s small, tightly held electrons → no compounds formed.
