CIE iGCSE Chemistry Paper 2 Prediction - 2025
CIE iGCSE Chemistry Paper 2 Prediction – 2025
Preparing for your IGCSE exam can be daunting, but with the right approach, you can achieve your goals with CIE iGCSE Chemistry Paper 2 Prediction
Ace your iGCSE exam! Find exam-style questions, detailed notes, and helpful resources to boost your understanding
iGCSE Practice Questions, Past Papers , Flashcards and notes available for iGCSE Students at IITian Academy.
Questions 1
Sodium chloride is a liquid at 900°C. Which row describes the arrangement and the motion of the particles in sodium chloride at 900°C?
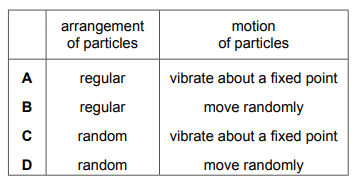
▶️Answer/Explanation
Ans D
Questions 2
Hydrogen chloride gas, HCl, reacts with ammonia gas, \(NH_3\), to form solid ammonium chloride. The apparatus is set up as shown. After a few minutes, a white cloud of solid ammonium chloride forms where the two gases meet.
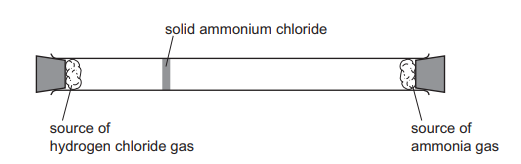
The experiment is repeated using hydrogen bromide gas, HBr, in place of hydrogen chloride. How far along the tube does the white cloud of solid ammonium bromide form? 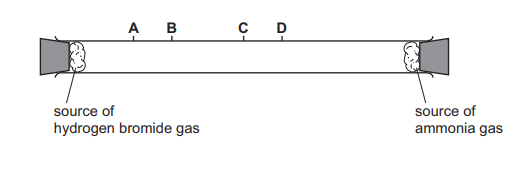
▶️Answer/Explanation
Ans A
Questions 3
Which gas has the slowest rate of diffusion?
A \(H_2\)
B \(NH_3\)
C \(CH_4\)
D \(CO_2\)
▶️Answer/Explanation
Ans D
Questions 4
Which statement explains why sulfur, S, has similar chemical properties to selenium, Se?
A They both have the same number of electrons in their outer electron shell.
B They are both solids at room temperature and pressure.
C They are both non-metals.
D They both form negative ions
▶️Answer/Explanation
Ans A
Questions 5
Substances P and Q both conduct electricity. P is a mixture of two different types of atom. Q is made of only one type of atom. Which row describes P and Q?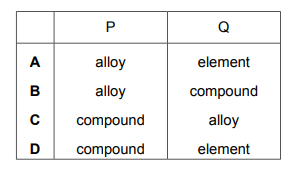
▶️Answer/Explanation
Ans A
Questions 6
Which statements about isotopes are correct?
1 Isotopes are atoms of different elements with the same number of protons.
2 Isotopes of the same element have the same chemical properties.
3 Isotopes are atoms with the same relative atomic mass.
4 Isotopes of the same element have the same electronic configuration.
A 1 and 2
B 1 and 3
C 2 and 4
D 3 and 4
▶️Answer/Explanation
Ans C
Questions 7
The structures of three substances are shown.
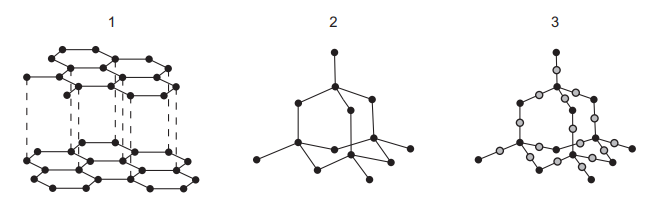
Which substances are hard and have a high melting point?
A 1, 2 and 3
B 1 only
C 2 only
D 2 and 3 only
▶️Answer/Explanation
Ans D
Questions 8
Which molecule contains a double covalent bond between two atoms of the same element?
A carbon dioxide
B ethanol
C ethene
D nitrogen
▶️Answer/Explanation
Ans C
Questions 9
How many electrons are shared in one molecule of nitrogen and in one molecule of ethene?
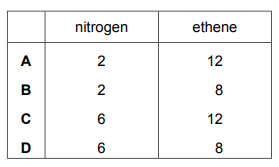
▶️Answer/Explanation
Ans C
Questions 10
The equation for the reaction of iron(III) oxide with carbon monoxide is shown.
\(Fe_2O_3 + 3CO → 2Fe + 3CO_2\)
What is the percentage yield of iron when 16.8 g of carbon monoxide reacts completely with iron(III) oxide to form 8.96g of iron?
A 26.7%
B 40.0%
C 53.3%
D 80.0%
▶️Answer/Explanation
Ans B
Questions 11
The incomplete equation for photosynthesis is shown.
![]()
Compound P is a product of the reaction. Which row describes the values of w, x and y and gives the empirical formula of compound P? 
▶️Answer/Explanation
Ans A
Questions 12
The concentration and volume of an aqueous alkali are known. Which additional information is required to calculate the number of moles of acid needed to neutralise the aqueous alkali?
A the concentration of the acid
B the equation for the acid–alkali reaction
C the formula of the acid
D the volume of the acid required for neutralisation
▶️Answer/Explanation
Ans B
Questions 13
Aqueous copper(II) sulfate is electrolysed using copper electrodes.
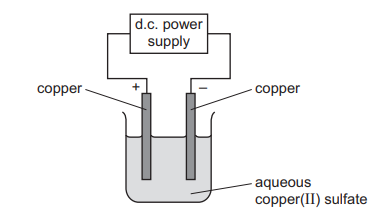
Which statement describes what happens during the electrolysis?
A Copper atoms gain electrons at the cathode and copper(II) ions lose electrons at the anode.
B Electrons move in the external circuit from the positive electrode to the negative electrode.
C Copper(II) ions move through the electrolyte from the cathode to the anode.
D Copper is formed at the cathode and oxygen is formed at the anode.
▶️Answer/Explanation
Ans B
Questions 14
Which statement about electrolysis is correct?
A Electrons move through the electrolyte from the cathode to the anode.
B Electrons move in the external circuit towards the cathode.
C Negative ions move in the external circuit towards the anode.
D Positive ions move through the electrolyte towards the anode.
▶️Answer/Explanation
Ans B
Questions 15
Plant cells use energy from sunlight for photosynthesis. Which row describes and explains the energy change that occurs?
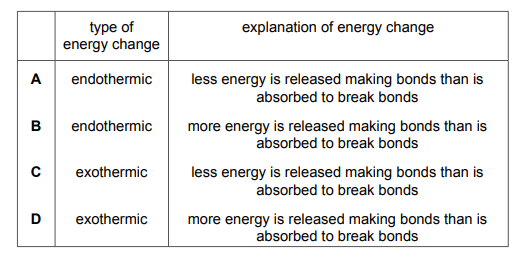
▶️Answer/Explanation
Ans A
Questions 16
The reaction pathway diagram for an endothermic reaction is shown. u, v and w are known energy values
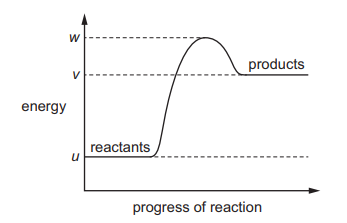
Which diagram shows the reaction pathway diagram when a catalyst is used in the reaction? 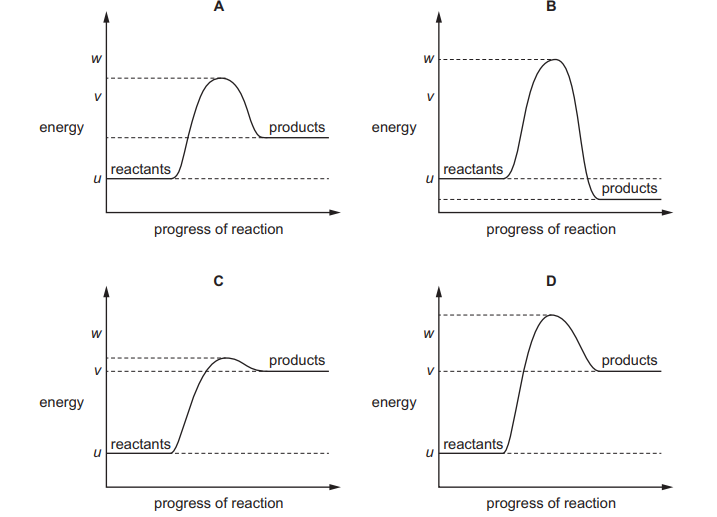
▶️Answer/Explanation
Ans C
Questions 17
In experiment 1, small lumps of limestone are added to dilute ethanoic acid at 40°C. The volume of carbon dioxide released is measured at regular time intervals. A graph of the results is shown.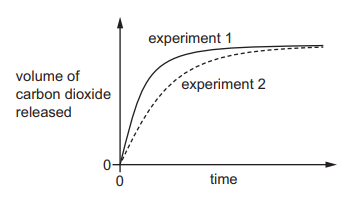
Which changes give the results shown in experiment 2?
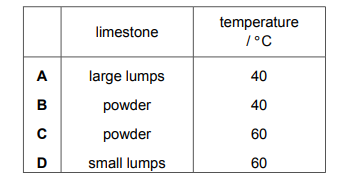
▶️Answer/Explanation
Ans A
Questions 18
A student adds excess magnesium ribbon to \(10 cm^3\) of 0.5 mol / \(dm^3\) sulfuric acid. The hydrogen gas produced is collected and its total volume is measured every 10 seconds.
The experiment is repeated with \(5 cm^3\) of 0.5 mol / \(dm^3\) sulfuric acid added to \(5 cm^3\) of water using the same mass of magnesium ribbon. Which line on the graph shows the results of the second experiment?
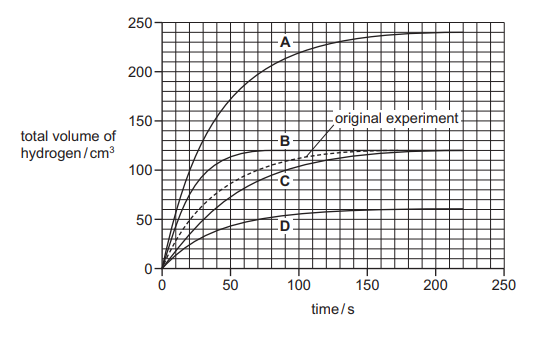
▶️Answer/Explanation
Ans D
Question 19
How does increasing the concentration affect the reacting particles in a chemical reaction?
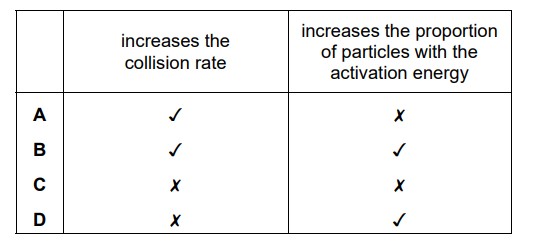
Answer/Explanation
Ans:
A
Questions 20
The Contact process is used to convert sulfur dioxide to sulfur trioxide. Vanadium(V) oxide is the catalyst in this process.
![]()
The forward reaction in this equilibrium is exothermic. Which statements about this process are correct?
1 The catalyst increases the rate of both the forward and backward reactions.
2 A low pressure increases the yield of sulfur trioxide.
3 A low pressure is used to keep the costs low.
4 A high temperature increases the yield of sulfur trioxide.
A 1 and 2
B 1 and 3
C 2 and 4
D 3 and 4
▶️Answer/Explanation
Ans B
Questions 21
Which row identifies the pressure and the catalyst used for the conversion of sulfur dioxide to sulfur trioxide in the Contact process?
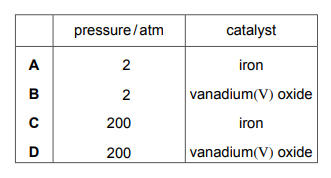
▶️Answer/Explanation
Ans B
Questions 22
The equation for a redox reaction is shown.
![]()
Potassium iodide is …… 1 …… agent in this reaction because iodide ions …… 2 …… electrons.
Which words complete gaps 1 and 2?
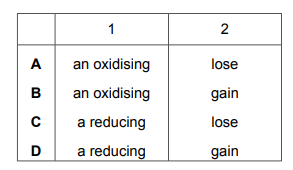
▶️Answer/Explanation
Ans C
Question 23
Aqueous iron(III) chloride, FeCl3, reacts with aqueous potassium iodide, KI.
vFeCl3 + wKI → xFeCl2 + yKCl + I2
Which statements are correct?
- In the balanced equation, v, w, x and y have the same value.
- Potassium iodide is an oxidizing agent.
- A dark brown solution is produced in the reaction.
A 1 and 2 only B 1 and 3 only C 2 and 3 only D 1, 2 and 3
Answer/Explanation
Ans:
B
Questions 24
Two acids, P and Q, with the same concentration and volume are reacted separately with the same mass of magnesium ribbon. The reactions produce the same total volume of hydrogen gas but acid Q reacts much more slowly than acid P. Which explanation for the difference between P and Q is correct?
A Acid P has a higher pH than acid Q.
B Acid P has a lower concentration of hydrogen ions.
C Acid Q is partially dissociated and acid P is fully dissociated.
D Acid Q is a proton acceptor
▶️Answer/Explanation
Ans C
Questions 25
Which statement describes the properties of hydrochloric acid?
A Carbon dioxide is produced when limestone reacts with hydrochloric acid.
B Hydrogen is produced when sodium hydroxide reacts with hydrochloric acid.
C Methyl orange turns yellow in strong hydrochloric acid.
D Red litmus paper turns blue when dipped into hydrochloric acid.
▶️Answer/Explanation
Ans A
Questions 26
Four statements about the reactions of oxides with dilute hydrochloric acid and with aqueous sodium hydroxide are listed.
1 Aluminium oxide reacts with both dilute hydrochloric acid and aqueous sodium hydroxide.
2 Calcium oxide reacts with both dilute hydrochloric acid and aqueous sodium hydroxide.
3 Copper(II) oxide reacts with dilute hydrochloric acid but not with aqueous sodium hydroxide.
4 Sulfur dioxide does not react with either dilute hydrochloric acid or aqueous sodium hydroxide.
Which statements are correct?
A 1 and 2
B 1 and 3
C 2 and 4
D 3 and 4
▶️Answer/Explanation
Ans B
Questions 27
Element E is in Group II of the Periodic Table. Which row describes element E and its oxide?
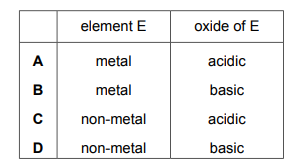
▶️Answer/Explanation
Ans B
Questions 28
Which statements describe the elements in Group VIII of the Periodic Table?
1 Their atoms have full outer electron shells.
2 They are unreactive metals.
3 They are monatomic gases.
4 They are diatomic gases.
A 1 and 3
B 1 and 4
C 2 and 3
D 2 and 4
▶️Answer/Explanation
Ans A
Questions 29
Which statements about transition elements are correct?
1 They have a low density.
2 They form ions with variable oxidation numbers.
3 They have a high melting point.
4 They form only colourless compounds.
A 1 and 2
B 1 and 4
C 2 and 3
D 3 and 4
▶️Answer/Explanation
Ans C
Questions 30
Which displayed formula represents the ester formed by the reaction of propan-1-ol with ethanoic acid?
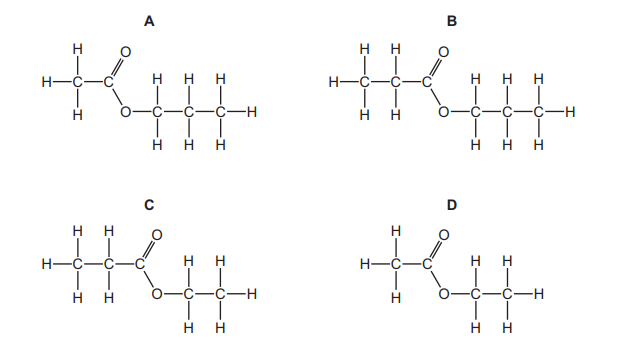
▶️Answer/Explanation
Ans A
Questions 31
Metal X acts as a sacrificial metal to prevent iron from corroding. Metal X does not act as a sacrificial metal to prevent aluminium from corroding. What is X?
A copper
B magnesium
C silver
D zinc
▶️Answer/Explanation
Ans D
Questions 32
Part of the reactivity series is shown.

Which metals are represented by X and Y? 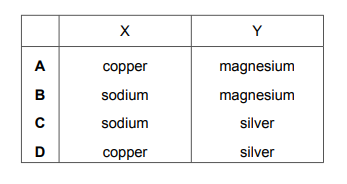
▶️Answer/Explanation
Ans B
Questions 33
Lead(II) iodide is formed as a precipitate in the reaction shown.
![]()
Which method is used to separate the lead(II) iodide from the mixture?
A crystallisation
B distillation
C evaporation
D filtration
▶️Answer/Explanation
Ans D
Question 34
The diagram shows the uses and treatment processes of muddy river water.

Which row identifies uses 1 and 2 and processes 1 and 2?

Answer/Explanation
Ans:
D
Questions 35
Oxides of nitrogen are produced by car engines. In a catalytic converter oxides of nitrogen are removed by reacting them with compound X. Which row describes the type of reaction oxides of nitrogen undergo and identifies compound X?
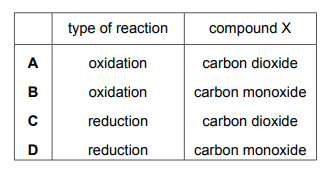
▶️Answer/Explanation
Ans D
Questions 36
Ethanol can be manufactured by fermentation and by the catalytic addition of steam to ethene. Which statement identifies an advantage of using one of these methods?
A Catalytic addition requires a high temperature and pressure.
B Ethanol produced by fermentation is extracted by distillation.
C Fermentation is a batch process.
D The raw material in fermentation is a renewable resource.
▶️Answer/Explanation
Ans D
Questions 37
The structure of a polymer is shown.
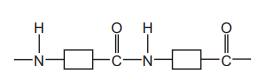
Which statement about this polymer is correct?
A Alkenes are polymerised to make the polymer.
B It is a polyester.
C It is an addition polymer.
D Water is produced when the polymer is made.
▶️Answer/Explanation
Ans D
Questions 38
Which structures represent a pair of structural isomers?
1 \(CH_3CH_2CH_2CH_2CH_2CH_3\)
2 \(CH_3CH_2CH_2CH_2CH(CH_3)_2\)
3 \(CH_3CH(CH_3)CH_2CH_2CH_3\)
4 \(CH_3CH(CH_3)CH_2CH_2CH_2CH_3\)
A 1 and 2
B 1 and 3
C 2 and 4
D 3 and 4
▶️Answer/Explanation
Ans B
Questions 39
Alkanes are a homologous series of hydrocarbons. The table shows the names and boiling points of the first four members of this series.
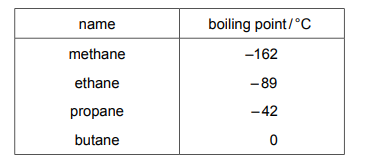
Pentane is the next member of the series. Which row gives the molecular formula and the boiling point of pentane? 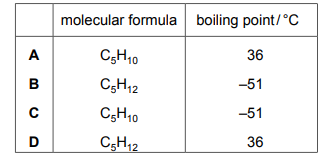
▶️Answer/Explanation
Ans D
Questions 40
Four pure substances, P, Q, R and S, are tested using chromatography. The same solvent is used each time. The table shows the distance moved by each substance and by the solvent from the baseline.
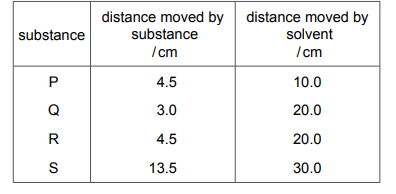
Which two substances are identical?
A P and R
B P and S
C Q and R
D Q and S
▶️Answer/Explanation
Ans B
