Rate of reaction- CIE iGCSE Chemistry Notes - New Syllabus
Rate of reaction for iGCSE Chemistry Notes
Core Syllabus
- Describe the effect on the rate of reaction of:
(a) changing the concentration of solutions
(b) changing the pressure of gases
(c) changing the surface area of solids
(d) changing the temperature
(e) adding or removing a catalyst, including enzymes - State that a catalyst increases the rate of a reaction and is unchanged at the end of a reaction
- Describe practical methods for investigating the rate of a reaction including change in mass of a reactant or a product and the formation of a gas
- Interpret data, including graphs, from rate of reaction experiments
Supplement Syllabus
- Describe collision theory in terms of:
(a) number of particles per unit volume
(b) frequency of collisions between particles
(c) kinetic energy of particles
(d) activation energy, $E_a$ - Describe and explain the effect on the rate of reaction of:
(a) changing the concentration of solutions
(b) changing the pressure of gases
(c) changing the surface area of solids
(d) changing the temperature
(e) adding or removing a catalyst, including enzymes using collision theory - State that a catalyst decreases the activation energy, $E_a$, of a reaction
- Evaluate practical methods for investigating the rate of a reaction including change in mass of a reactant or a product and the formation of a gas
Factors Affecting the Rate of Reaction
Factors Affecting the Rate of Reaction
The rate of a chemical reaction refers to how quickly reactants are converted into products. Several factors influence how fast or slow a reaction occurs:
(a) Concentration of Solutions
Increasing the concentration of a reactant in solution increases the number of particles per unit volume. This leads to more frequent collisions between reactant particles, which increases the rate of reaction.

Example
What is the effect of concentration of HCl on the reaction during hydrochloric acid reacting with magnesium ribbon?
▶️Answer/Explanation
\( \text{Mg (s)} + 2\text{HCl (aq)} \rightarrow \text{MgCl}_2 \text{(aq)} + \text{H}_2 \text{(g)} \)
Higher concentration of HCl leads to faster production of hydrogen gas because collisions occur more frequently.
(b) Pressure of Gases
Increasing the pressure of a gas compresses the particles into a smaller volume. This increases the number of gas particles per unit volume, so collisions between particles happen more often, increasing the reaction rate.
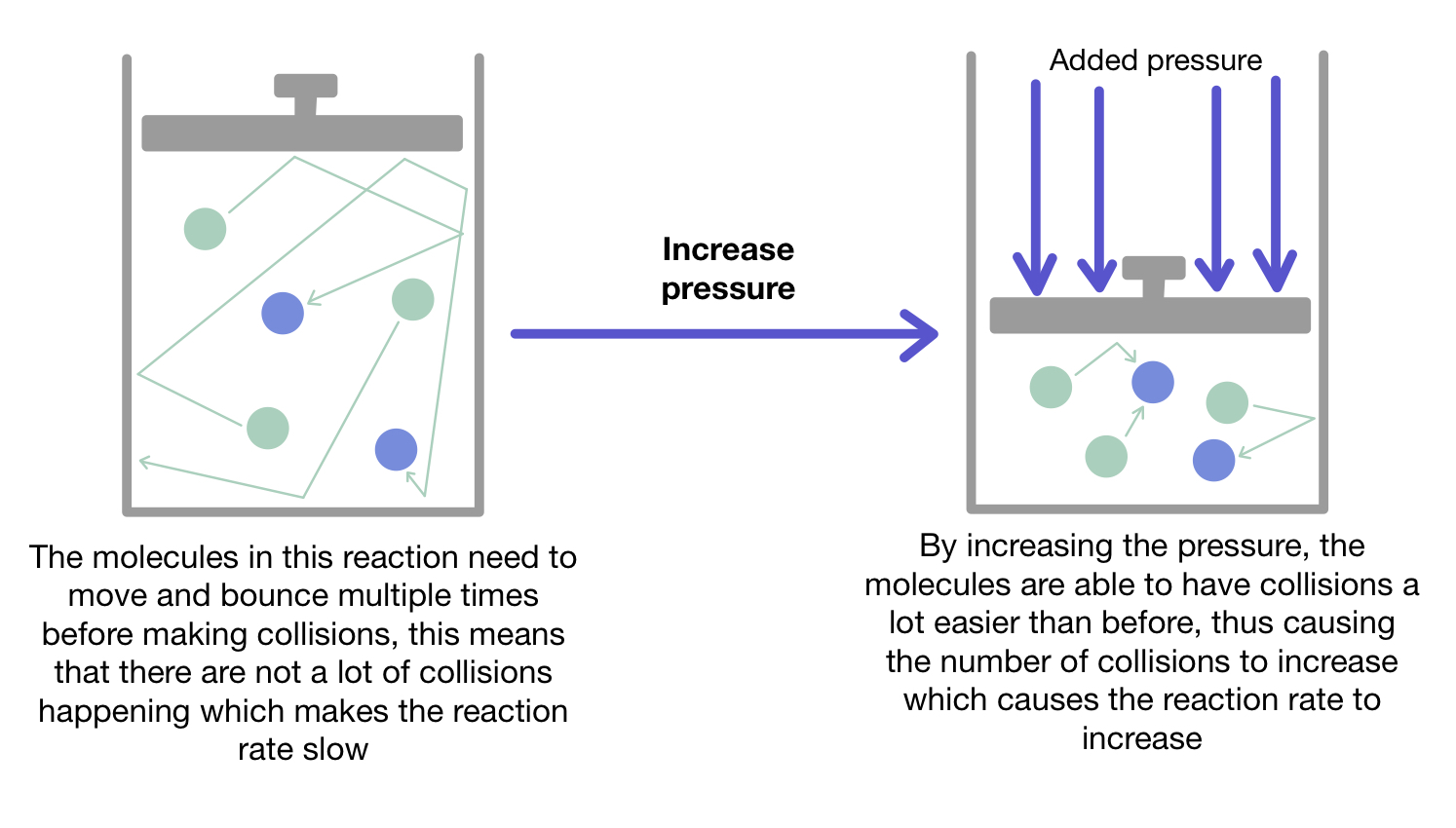
Example
What is the effect of pressure during the reaction between hydrogen and oxygen?
▶️Answer/Explanation
\( 2\text{H}_2 + \text{O}_2 \rightarrow 2\text{H}_2\text{O} \)
Increasing the pressure increases the rate at which these gas molecules collide and react.
(c) Surface Area of Solids
Breaking a solid into smaller pieces increases its surface area. More particles of the solid are exposed and available for collisions with the other reactant, increasing the rate of reaction.
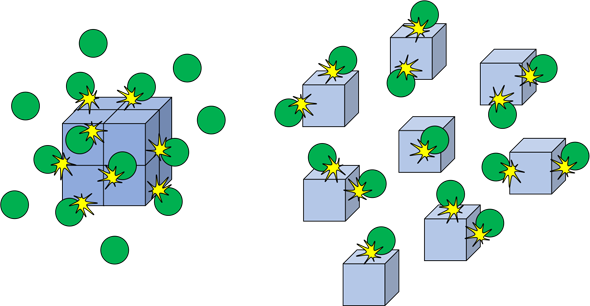
Example
What is the effect of surface area of calcium carbonate during its reaction with hydrochloric acid?
▶️Answer/Explanation
\( \text{CaCO}_3 \text{(s)} + 2\text{HCl (aq)} \rightarrow \text{CaCl}_2 \text{(aq)} + \text{H}_2\text{O (l)} + \text{CO}_2 \text{(g)} \)
Powdered calcium carbonate reacts faster than large marble chips because of a greater surface area.
(d) Temperature
Raising the temperature increases the kinetic energy of the particles. This makes them move faster, increasing the frequency of collisions and making more of them energetic enough to overcome the activation energy barrier, thus speeding up the reaction.
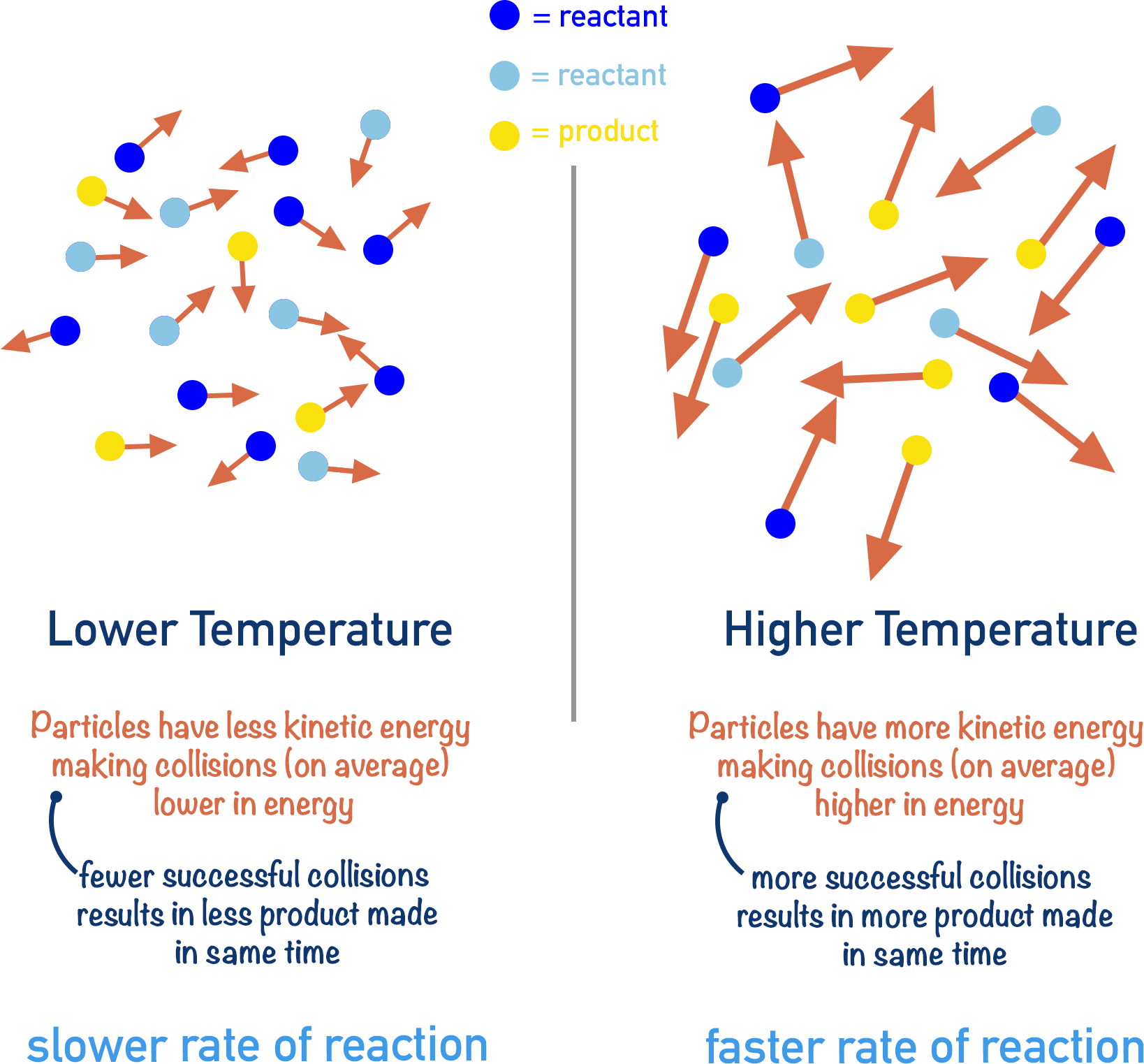
Example
What is the effect of temperature on sodium thiosulfate and hydrochloric acid reaction?
▶️Answer/Explanation
\( \text{Na}_2\text{S}_2\text{O}_3 \text{(aq)} + 2\text{HCl (aq)} \rightarrow 2\text{NaCl (aq)} + \text{SO}_2 \text{(g)} + \text{S (s)} + \text{H}_2\text{O (l)} \)
The reaction is faster at higher temperatures — the sulfur precipitate forms more quickly and clouds the solution sooner.
(e) Catalysts (Including Enzymes)
A catalyst increases the rate of a reaction by providing an alternative reaction pathway with lower activation energy. Catalysts are not consumed in the reaction and can be reused.
Example
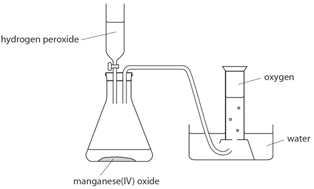
What is the effect of catalysts on the decomposition of hydrogen peroxide using manganese(IV) oxide?
▶️Answer/Explanation
\( 2\text{H}_2\text{O}_2 \rightarrow 2\text{H}_2\text{O} + \text{O}_2 \)
This reaction is very slow without a catalyst. Adding \( \text{MnO}_2 \) greatly speeds up the reaction by lowering the activation energy.
Role of Catalysts in Reactions
Role of Catalysts in Reactions
A catalyst is a substance that increases the rate of a chemical reaction without itself being used up or chemically changed at the end of the reaction.
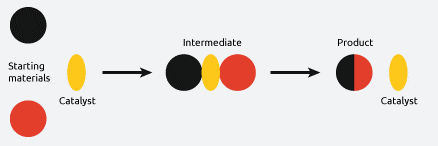
Key Features of Catalysts:
- They lower the activation energy of a reaction.
- They provide an alternative pathway for the reaction to occur.
- They do not alter the chemical equilibrium or the overall enthalpy change (\( \Delta H \)).
- Catalysts remain chemically unchanged after the reaction — they are not consumed.
Importance of Catalysts:
- Used to speed up reactions in industrial processes (e.g. Haber process, catalytic converters).
- Make reactions more economical by reducing energy requirements.
- Help reduce environmental impact by lowering temperatures and pressures needed.
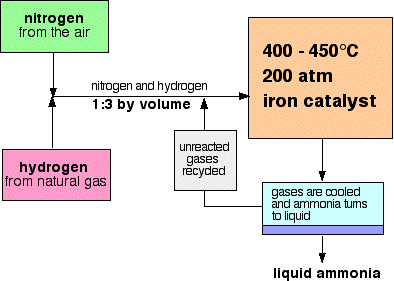
Example
What is the effect of catalyst on the reaction during the Haber Process?
▶️Answer/Explanation
\( \text{N}_2 + 3\text{H}_2 \rightleftharpoons 2\text{NH}_3 \)
Iron catalyst is used to increase the rate of ammonia formation at moderate temperatures and pressures.
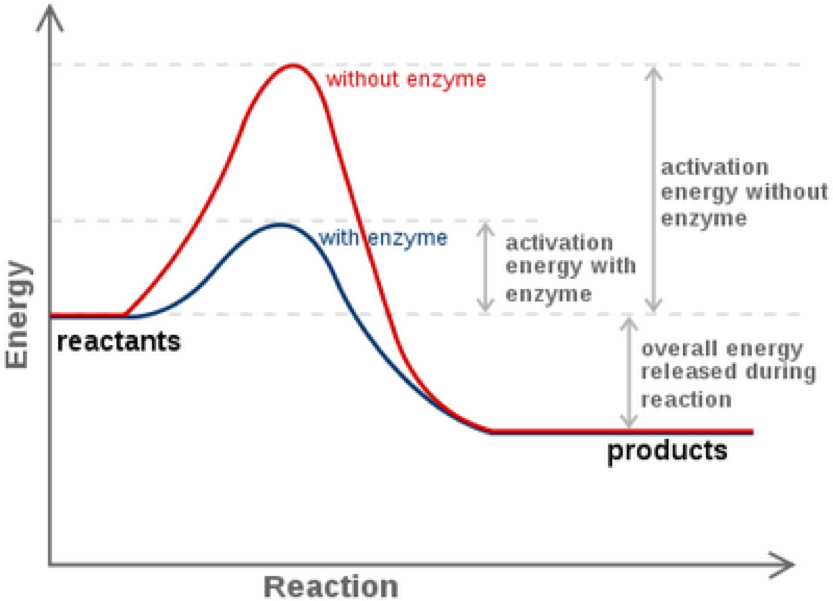
Effect of Catalyst on Activation Energy
A catalyst works by providing an alternative pathway for the reaction with a lower activation energy.
Activation Energy (\( E_a \)):
Activation energy is the minimum energy that reacting particles must have for a successful collision that leads to a reaction.
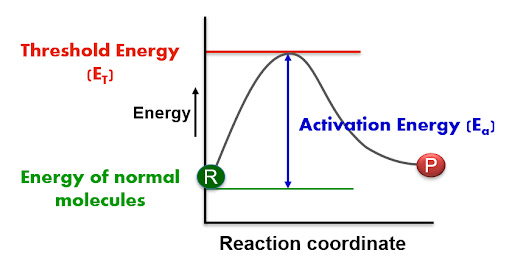
Effect of a Catalyst on Activation Energy:
- Catalysts lower the activation energy (\( E_a \)) of a reaction.
- This means more reacting particles have enough energy to overcome the energy barrier.
- As a result, the number of successful collisions increases → the reaction becomes faster.
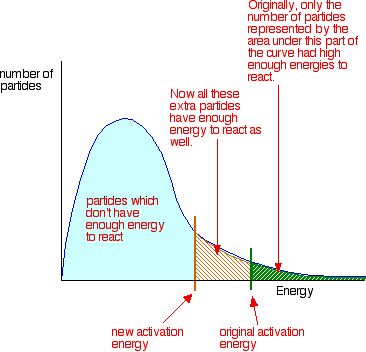
Important Notes:
- Catalysts do not change the enthalpy change (\( \Delta H \)) of the reaction.
- They are not used up during the reaction and can be reused.
- Different reactions require specific catalysts.
- Enzymes are biological catalysts that work in living systems.
Example
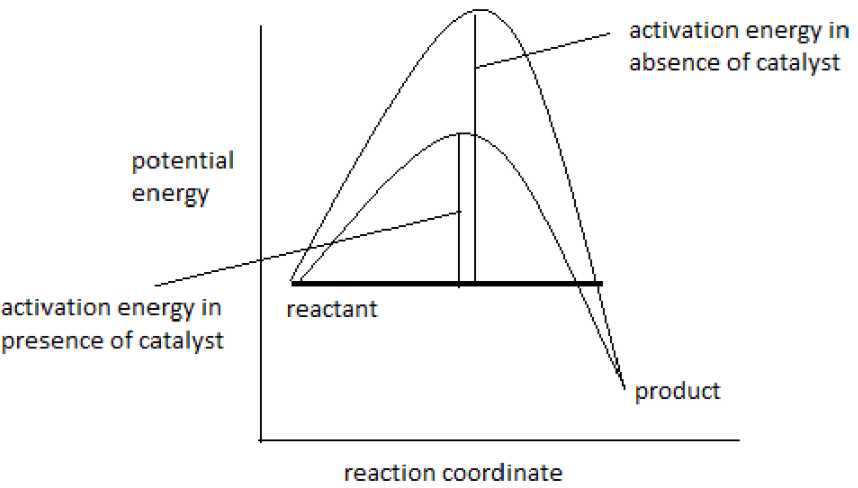
Give a visual diagram showing effect of catalyst on activation energy.
▶️Answer/Explanation
A reaction profile diagram shows:
- Reactants at a higher energy level
- Products at a lower energy level (for exothermic reaction)
- Activation energy with and without catalyst
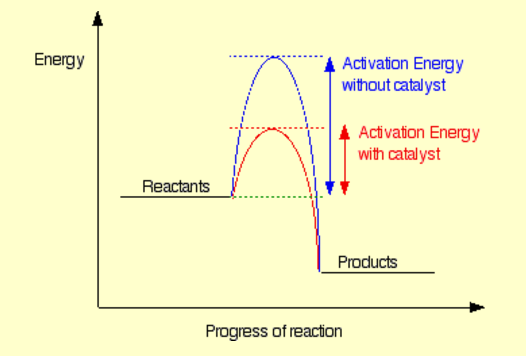
The catalyzed reaction has a smaller ‘hump’, showing that less energy is needed for the reaction to proceed.
Practical Methods for Investigating the Rate of Reaction
Rate of a reaction
The rate of a chemical reaction is a measure of how fast the reactants are converted into products. It can be observed by measuring how quickly a product is formed or how quickly a reactant is used up over time.
Common Methods of Investigating Reaction Rate:
1. Measuring Volume of Gas Produced
This is suitable for reactions where a gas is one of the products.
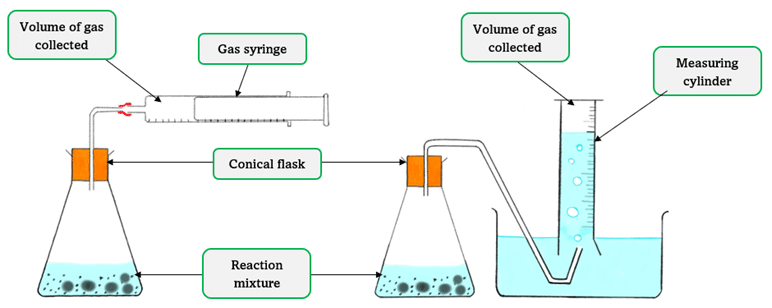
Example Reaction:
\( \text{Mg}_{(s)} + 2\text{HCl}_{(aq)} \rightarrow \text{MgCl}_2{}_{(aq)} + \text{H}_2{}_{(g)} \)
Method:
- Add magnesium ribbon to hydrochloric acid in a conical flask.
- Collect the hydrogen gas in a gas syringe and measure the volume at fixed time intervals.
Advantages:
- Gives quantitative data.
- Easy to record and plot on a graph.
Disadvantages:
- Gas syringe must be airtight to prevent leaks.
- Not suitable for very slow reactions that produce tiny gas volumes.
2. Measuring Change in Mass
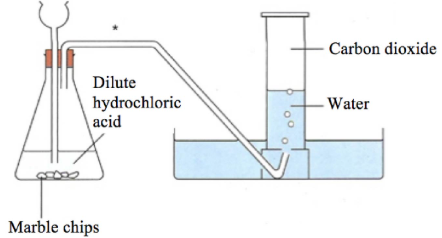
This is suitable when a gas escapes from the reaction vessel, causing a decrease in mass.
Example Reaction:
\( \text{CaCO}_3{}_{(s)} + 2\text{HCl}_{(aq)} \rightarrow \text{CaCl}_2{}_{(aq)} + \text{H}_2\text{O}_{(l)} + \text{CO}_2{}_{(g)} \)
Method:
- Place the reaction flask on a digital balance.
- Start the reaction and record the loss in mass at regular intervals.
Advantages:
- Simple to set up and sensitive to mass changes.
- Does not require special gas collection equipment.
Disadvantages:
- Open system – gas escapes into the air (environmental concern for toxic gases).
- Air currents may affect the balance readings.
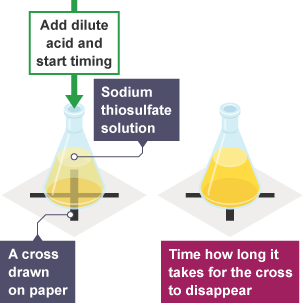
3. Disappearing Cross / Colour Change Method
Used when the reaction mixture becomes cloudy or changes colour.
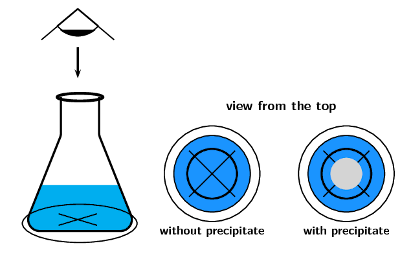
Example Reaction:
\( \text{Na}_2\text{S}_2\text{O}_3{}_{(aq)} + 2\text{HCl}_{(aq)} \rightarrow 2\text{NaCl}_{(aq)} + \text{SO}_2{}_{(g)} + \text{S}_{(s)} + \text{H}_2\text{O}_{(l)} \)
Method:
- Place a piece of paper with a black cross under the reaction flask.
- Observe the time taken for the cross to disappear as a yellow precipitate of sulfur forms.
Advantages:
- Simple and inexpensive.
- Does not require specialist equipment.
Disadvantages:
- Subjective – depends on human judgement.
- Less accurate and not suitable for quantitative analysis.
4. Measuring pH Change (for acid-base reactions)
For reactions involving acids or bases, pH meters or indicators can be used.
Method:
- Use a pH probe connected to a data logger or record pH manually at regular intervals.
Advantages:
- Useful for monitoring neutralization reactions.
- Can be automated using data loggers.
Disadvantages:
- Limited to acid-base reactions.
- Less suitable for fast reactions.
Example
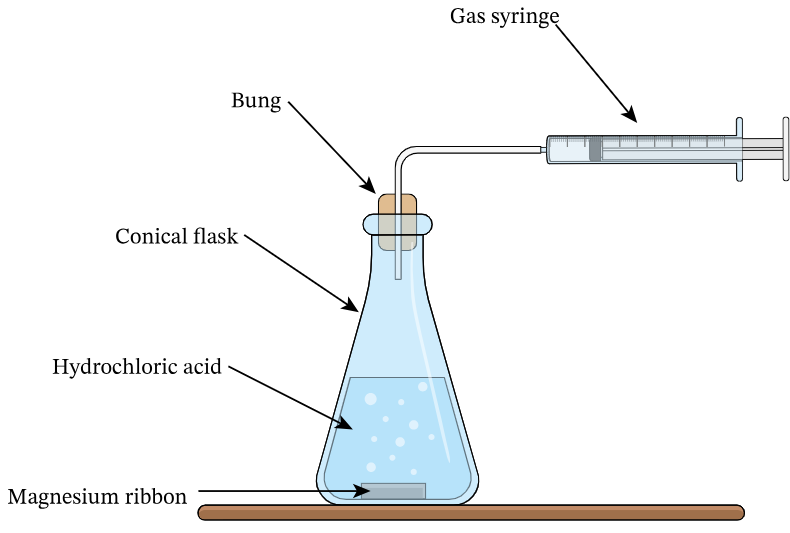
In the reaction between magnesium ribbon and hydrochloric acid, describe rate of reaction.
▶️Answer/Explanation
Hydrogen gas is produced:
\( \text{Mg (s)} + 2\text{HCl (aq)} \rightarrow \text{MgCl}_2 \text{(aq)} + \text{H}_2 \text{(g)} \)
1. Place magnesium ribbon into dilute hydrochloric acid in a conical flask.
2. Collect the gas using a gas syringe.
3. Record gas volume every 10 seconds.
4. Plot gas volume vs time.
5. Compare rate of reaction using different acid concentrations.
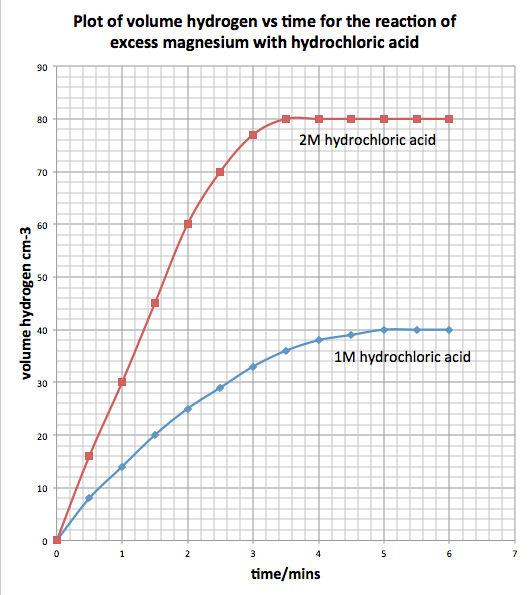
Interpreting Data from Rate of Reaction Experiments
How to Process Data:
Data is often represented using a graph:
- x-axis: Time (s)
- y-axis: Amount of product formed or reactant used (e.g., volume of gas in cm³ or mass in g)
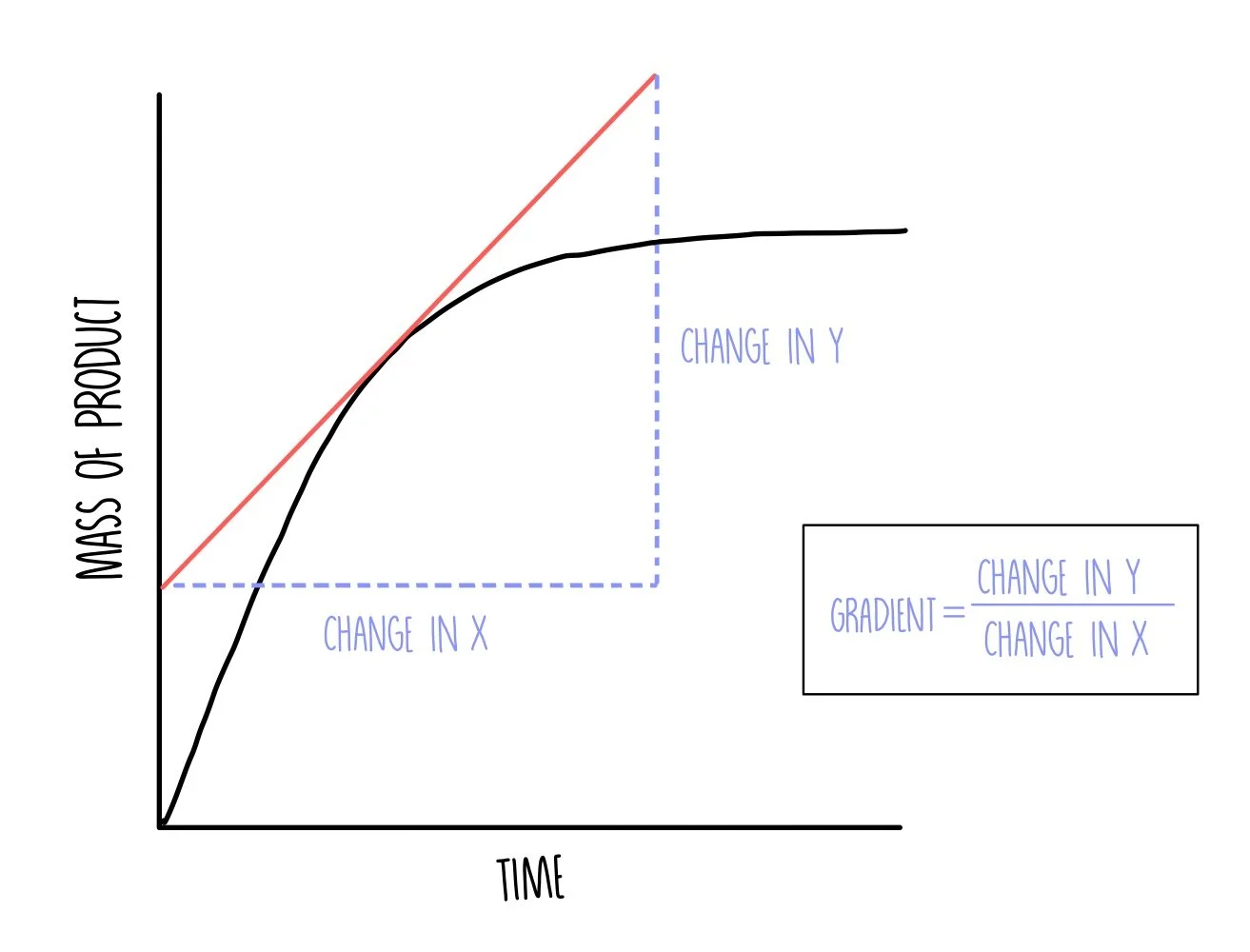
Key Features of the Graph:
- Initial Slope (Gradient): Shows the initial rate of reaction (steeper slope = faster rate)
- Curve Leveling Off: Indicates the reaction is slowing down as reactants are used up
- Flat Line: Reaction has stopped (all reactants used up)
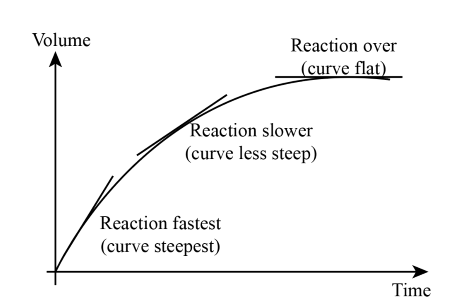
How to Compare Graphs:
- If the curve reaches the same final value but at different speeds – same amount of product formed but different rates
- If the curve reaches a different final value – different amount of product formed (indicates different amounts or concentrations of reactants)
Interpreting Graph Shapes:
- Steeper Slope: Faster reaction (due to higher temperature, higher concentration, larger surface area, or catalyst)
- Shallower Slope: Slower reaction
- Leveling off: Reaction completed.
Example Graph Analysis:
Suppose you’re investigating the rate of reaction between marble chips (calcium carbonate) and hydrochloric acid:
\( \text{CaCO}_3{}_{(s)} + 2\text{HCl}_{(aq)} \rightarrow \text{CaCl}_2{}_{(aq)} + \text{H}_2\text{O}_{(l)} + \text{CO}_2{}_{(g)} \)
Two graphs are plotted:
- Graph A: Uses smaller marble chps → steep initial slope → fast reaction
- Graph B: Uses large marble chips → shallow initial slope → slower reaction
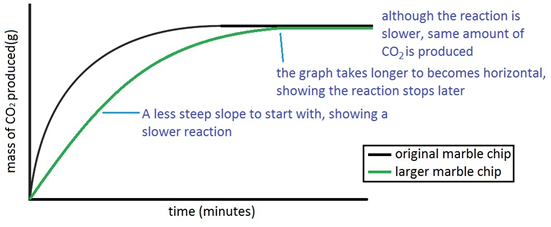
Both curves eventually level off at the same volume of gas → indicates same amount of product formed, just at different speeds.
Example
Two students performed the same reaction at different temperatures. Compare rate of reactions.
▶️Answer/Explanation
Student A’s graph shows a steeper initial slope and reaches the plateau quicker. This means:
Student A’s reaction was faster — likely due to higher temperature, which increased the kinetic energy of particles and collision frequency.
Collision Theory
Collision Theory
Collision theory explains how chemical reactions occur and why their rates differ. According to this theory, particles must:
- Collide with each other
- Collide with sufficient energy (called activation energy, \( E_a \))
- Collide in the correct orientation
Factors Affecting the Rate Based on Collision Theory:
(a) Number of particles per unit volume (Concentration and Pressure)
- In solutions, increasing the concentration means more particles in the same volume.
- In gases, increasing the pressure compresses the gas, increasing the number of particles in the same space.
- Both lead to more frequent collisions and thus a faster reaction rate.
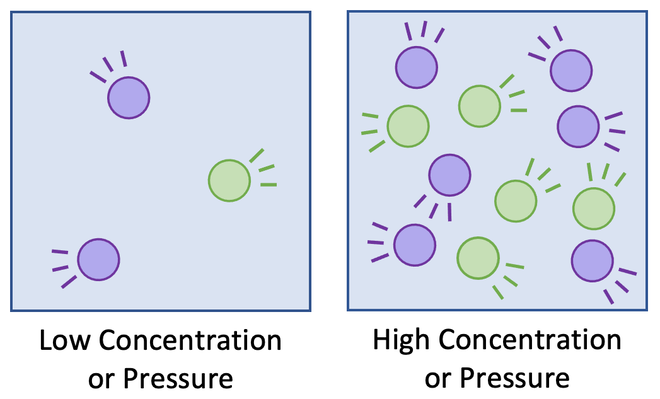
(b) Frequency of collisions
- The more often particles collide, the higher the chance that a successful collision will occur.
- Increasing concentration, pressure, or surface area increases the frequency of collisions.
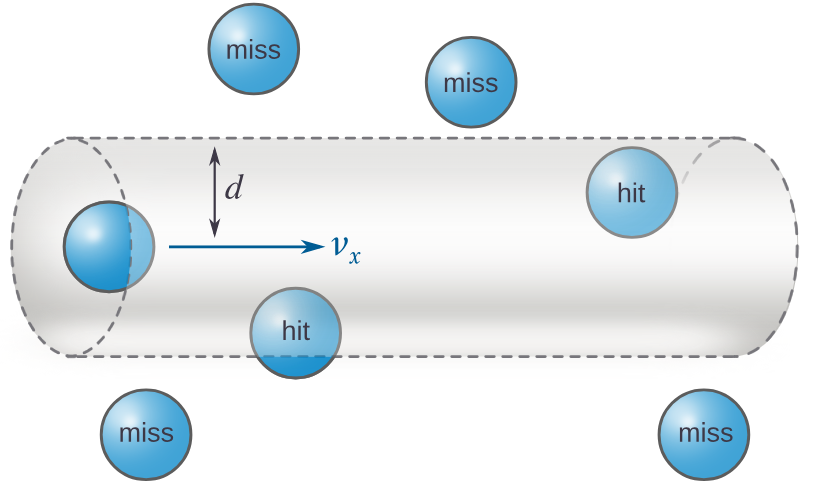
(c) Kinetic energy of particles (Temperature)
- Raising the temperature gives particles more energy and makes them move faster.
- This increases both the frequency of collisions and the proportion of particles with energy ≥ \( E_a \).
- Therefore, the rate of reaction increases significantly with temperature.
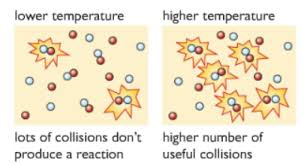
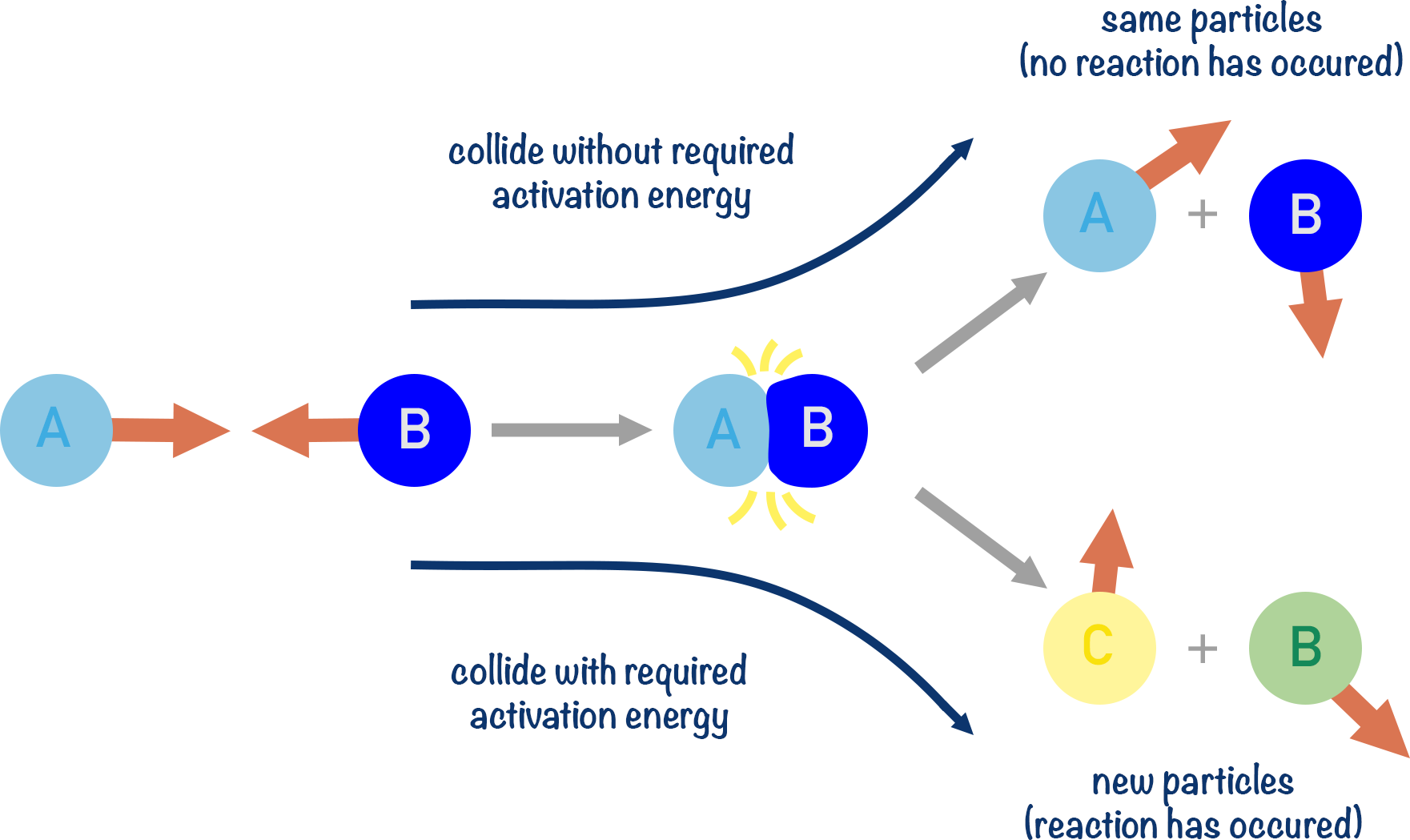
(d) Activation energy, \( E_a \)
- This is the minimum energy needed for a reaction to occur during a collision.
- If the energy of colliding particles is less than \( E_a \), the reaction will not happen.
- Catalysts lower \( E_a \), making more collisions successful.
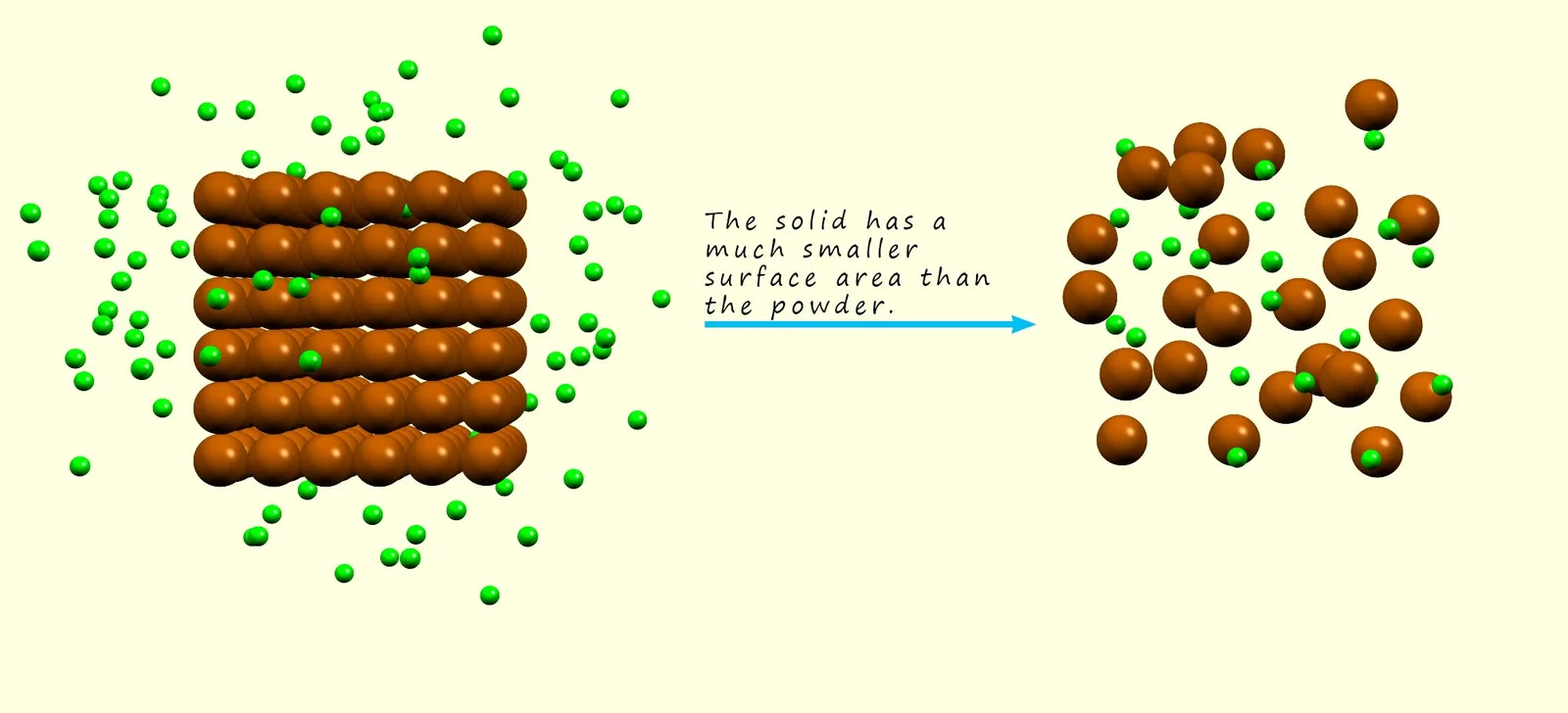
(e) Changing the surface area of solids
- Breaking a solid into smaller pieces or powder increases surface area.
- More particles are exposed to collisions with the other reactant.
- More surface area → more frequent collisions → faster rate.
(f) Adding or removing a catalyst (including enzymes)
- A catalyst provides an alternative reaction pathway with lower activation energy (\( E_a \)).
- This means more particles now have sufficient energy to react.
- Thus, more collisions are successful → reaction rate increases.
- Catalysts are not consumed in the reaction and can be reused.
| Factor | Effect on Particles | Resulting Effect on Rate |
|---|---|---|
| Concentration / Pressure | More particles per volume → more collisions | Rate increases |
| Temperature | More kinetic energy → faster movement | More frequent and energetic collisions → rate increases |
| Surface Area | More area exposed for collisions | Rate increases |
| Catalyst | Lowers activation energy | More successful collisions → rate increases |
Example
Why does a powdered solid react faster than a large lump of the same solid?
▶️Answer/Explanation
A powdered solid has a greater surface area exposed to the reactant particles in solution or gas.
This increases the frequency of collisions → higher chance of successful collisions → faster rate of reaction.
Example
Manganese(IV) oxide speeds up the decomposition of hydrogen peroxide. How?
▶️Answer/Explanation
\( \text{2H}_2\text{O}_2 \rightarrow \text{2H}_2\text{O} + \text{O}_2 \)
Manganese(IV) oxide lowers the activation energy → more successful collisions → oxygen is released faster.
