Reactivity series- CIE iGCSE Chemistry Notes - New Syllabus
Reactivity series for iGCSE Chemistry Notes
Core Syllabus
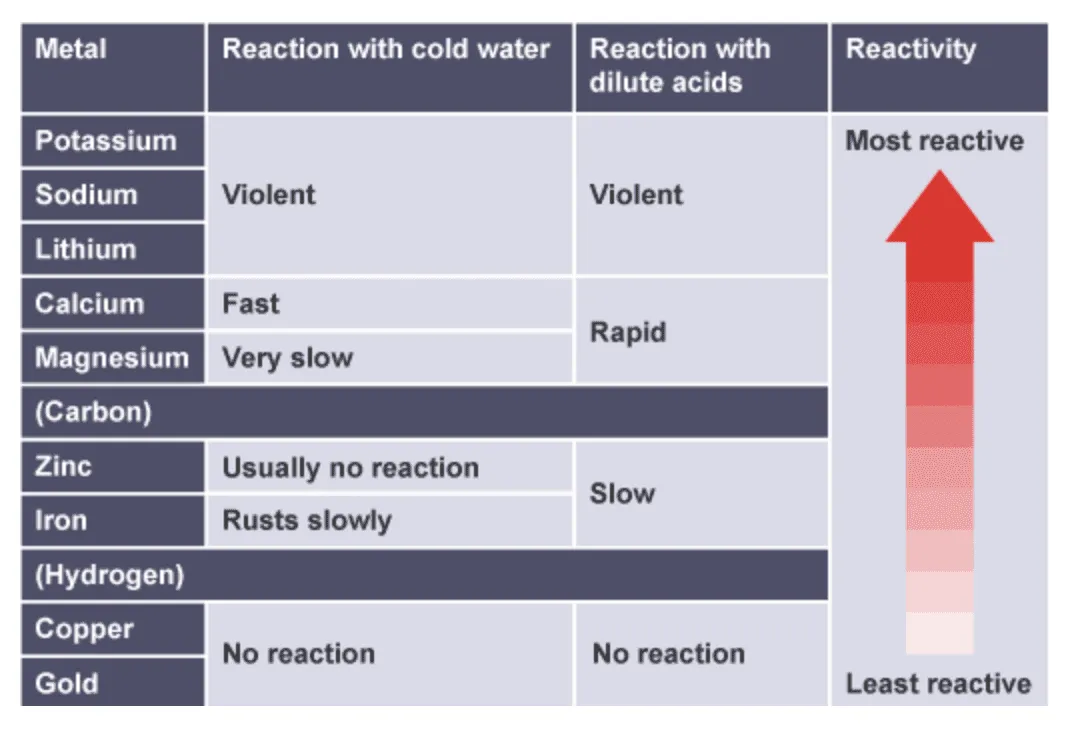
State the order of the reactivity series as: potassium, sodium, calcium, magnesium, aluminium, carbon, zinc, iron, hydrogen, copper, silver, gold
Describe the reactions, if any, of:
(a) potassium, sodium and calcium with cold water
(b) magnesium with steam
(c) magnesium, zinc, iron, copper, silver and gold with dilute hydrochloric acid
and explain these reactions in terms of the position of the metals in the reactivity seriesDeduce an order of reactivity from a given set of experimental results
Supplement Syllabus
- Describe the relative reactivities of metals in terms of their tendency to form positive ions, by displacement reactions, if any, with the aqueous ions of magnesium, zinc, iron, copper and silver
- Explain the apparent unreactivity of aluminium in terms of its oxide layer
The Reactivity Series
The Reactivity Series
The reactivity series is a list of metals (and a few non-metals like carbon and hydrogen) arranged in order of their reactivity, from the most reactive at the top to the least reactive at the bottom. It helps us predict how metals will react with water, steam, acids, and the ions of other metals in displacement reactions.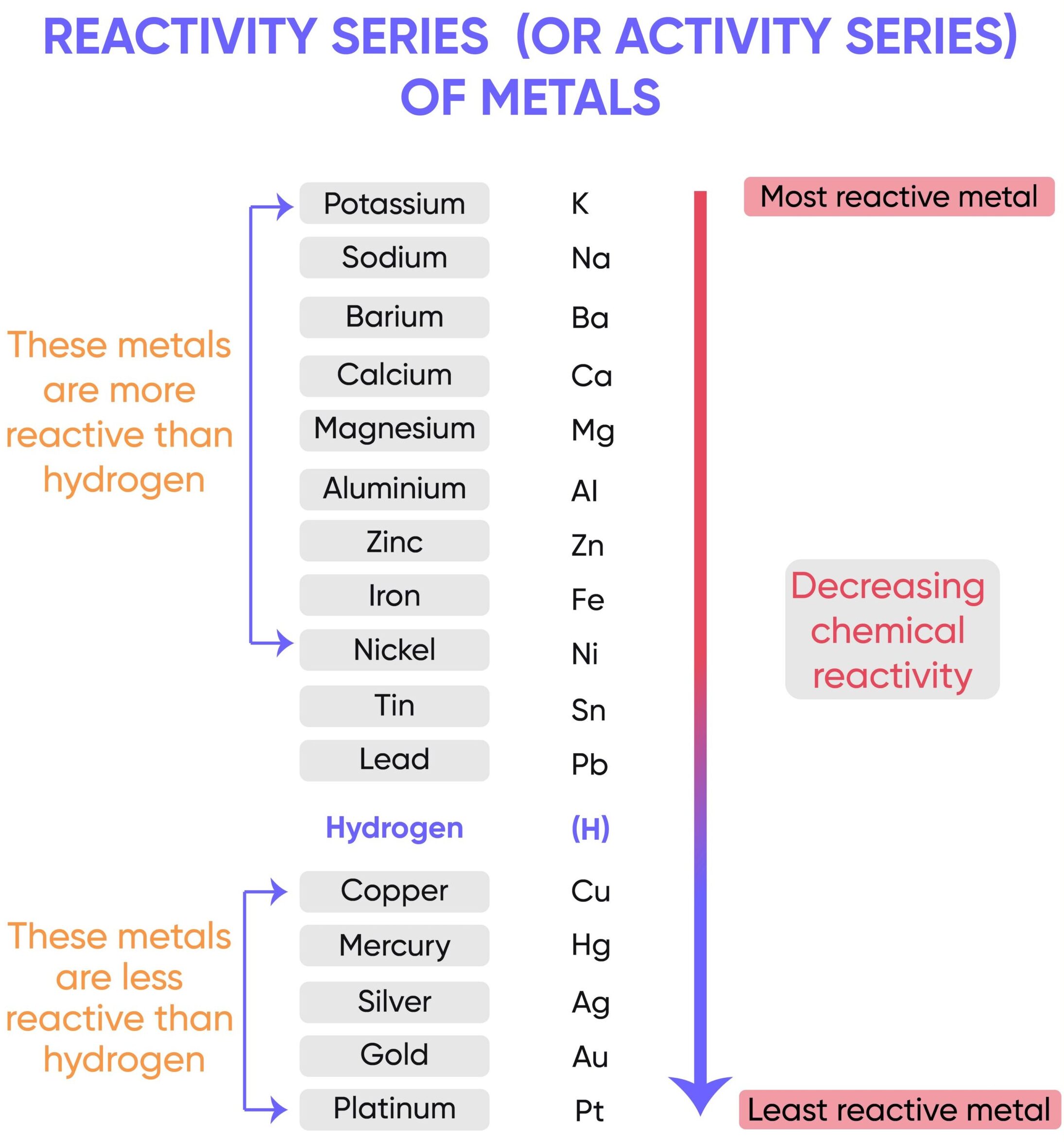
The order of the reactivity series
Potassium → Sodium → Calcium → Magnesium → Aluminium → Carbon → Zinc → Iron → Hydrogen → Copper → Silver → Gold
Explanation of positions
- Very reactive metals (K, Na, Ca) – these metals are at the top because they react violently with cold water and must be stored carefully.
- Moderately reactive metals (Mg, Al, Zn, Fe) – these metals do not react as vigorously with water, but they react with acids and in some cases with steam.
- Carbon and Hydrogen – included for comparison. Carbon can reduce some metal oxides, and hydrogen can be displaced from acids by more reactive metals.
- Less reactive metals (Cu, Ag, Au) – these are below hydrogen, meaning they cannot displace hydrogen from acids. They are much less reactive and some, like gold, are considered unreactive or “noble”.
Why this order matters
- It allows prediction of which metals can displace others in reactions.
- It explains why some metals can be extracted by reduction with carbon (those below carbon), while others require electrolysis (those above carbon).
- It helps in understanding everyday uses – for example, why copper and gold are used for jewellery and wiring (they do not corrode easily), while sodium and potassium are not used in construction (too reactive).
Example
Why is carbon included in the reactivity series even though it is not a metal?
▶️Answer/Explanation
Carbon is used in the extraction of metals from their oxides (e.g., zinc oxide, iron(III) oxide). Its position in the series helps determine whether a metal can be reduced by carbon in a blast furnace.
Deducing an order of reactivity from experimental results
The reactivity of metals can be determined by carrying out experiments and observing how vigorously they react with substances such as water, steam, acids, or metal salt solutions. From these results, the metals can be arranged in order of reactivity.
Methods of deducing reactivity
- Reaction with water – Highly reactive metals such as potassium, sodium, and calcium react with cold water, producing hydrogen gas and an alkali solution. Less reactive metals do not react with cold water but may react with steam, for example magnesium, zinc, and iron.
- Reaction with dilute acids – Metals above hydrogen in the reactivity series displace hydrogen gas from acids. The speed of effervescence and amount of heat released indicate relative reactivity. Metals below hydrogen, such as copper, silver, and gold, do not react.
- Displacement reactions – A more reactive metal displaces a less reactive metal from its salt solution. For example, zinc can displace copper from copper sulfate, but copper cannot displace zinc from zinc sulfate. These reactions clearly show which metal is more reactive.

General observations
- A metal that reacts vigorously with acids and water is placed higher in the series.
- A metal that reacts slowly or not at all is placed lower.
- If one metal displaces another from a salt solution, it is higher in the series than the displaced metal.
Example experiment
- If strips of magnesium, zinc, and copper are placed into dilute hydrochloric acid:
- Magnesium reacts rapidly, releasing many hydrogen bubbles.
- Zinc reacts moderately, producing fewer bubbles.
- Copper does not react at all.
From this, we deduce: Magnesium is more reactive than Zinc, and Zinc is more reactive than Copper. Therefore the order is Mg > Zn > Cu.
Example
Three metals A, B, and C are tested with water and dilute hydrochloric acid: A reacts violently with cold water, producing hydrogen gas. B does not react with water but reacts slowly with acid. C does not react with either water or acid.
▶️Answer/Explanation
Metal A must be very reactive (like sodium or calcium) since it reacts with cold water.
Metal B reacts only with acid, showing medium reactivity (like zinc or iron).
Metal C shows no reaction, meaning it is below hydrogen in the reactivity series (like copper or silver).
Order of reactivity: A → B → C.
Example
A student places magnesium ribbon into copper(II) sulfate solution. The blue solution fades and a brown solid forms on the ribbon.
▶️Answer/Explanation
The brown solid is copper, deposited as magnesium displaces copper from copper(II) sulfate.
This shows magnesium is more reactive than copper.
Therefore, order: Magnesium is above copper in the reactivity series.
Reactions of metals with water, steam, and acids
Reactions of metals with water, steam, and acids
The position of a metal in the reactivity series determines how it reacts with water, steam, and dilute acids. The more reactive a metal is, the more vigorously it reacts.
(a) Potassium, sodium, and calcium with cold water
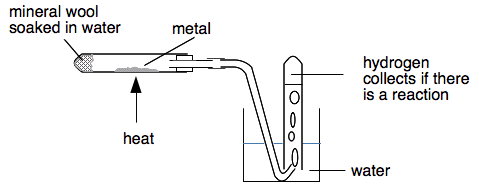
- Potassium reacts explosively with cold water, producing hydrogen gas and potassium hydroxide solution. A lilac flame is often observed as hydrogen ignites.
- Sodium reacts very vigorously with cold water, releasing hydrogen gas and forming sodium hydroxide solution. The reaction is less violent than potassium but still highly dangerous.
- Calcium reacts less violently with cold water, slowly releasing bubbles of hydrogen gas and forming calcium hydroxide solution, which is only slightly soluble (limewater).
General reaction: \( \text{Metal} + \text{Water} \rightarrow \text{Metal hydroxide} + \text{Hydrogen} \)
Example: \( 2\text{Na} + 2\text{H}_2\text{O} \rightarrow 2\text{NaOH} + \text{H}_2 \)
(b) Magnesium with steam
- Magnesium does not react with cold water but reacts with steam when heated strongly. It produces magnesium oxide and hydrogen gas.
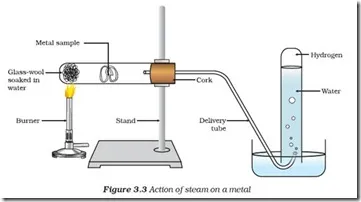
Equation: \( \text{Mg} + \text{H}_2\text{O (g)} \rightarrow \text{MgO} + \text{H}_2 \)
(c) Magnesium, zinc, iron, copper, silver, and gold with dilute hydrochloric acid
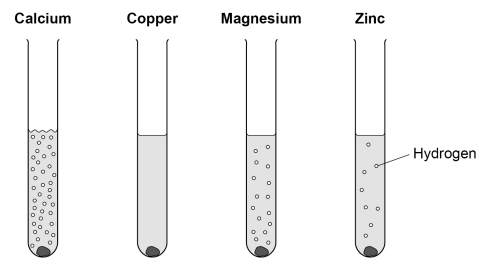
- Magnesium reacts rapidly with dilute hydrochloric acid, producing hydrogen gas and magnesium chloride solution.
- Zinc reacts moderately with dilute acid, releasing hydrogen gas steadily to form zinc chloride solution.
- Iron reacts slowly with dilute acid, producing hydrogen gas and iron chloride solution, faster if heated.
- Copper, silver, and gold show no reaction with dilute hydrochloric acid because they are below hydrogen in the reactivity series and cannot displace hydrogen from acids.
General reaction: \( \text{Metal} + \text{Acid} \rightarrow \text{Salt} + \text{Hydrogen} \)
Example: \( \text{Zn} + 2\text{HCl} \rightarrow \text{ZnCl}_2 + \text{H}_2 \)
Explanation in terms of reactivity series:
- Metals above hydrogen in the series can displace hydrogen from acids, so they react with hydrochloric acid to release hydrogen gas.
- Metals below hydrogen cannot displace it, so they show no reaction with acids.
- The higher up the metal is in the series, the faster and more vigorous its reaction with water, steam, or acid.
Example
Explain when a strip of zinc is placed in dilute hydrochloric acid. Bubbles of gas are seen.
▶️Answer/Explanation
\( \text{Zn} + 2\text{HCl} \rightarrow \text{ZnCl}_2 + \text{H}_2 \)
The effervescence is due to hydrogen gas. Zinc is above hydrogen in the reactivity series, so it can displace hydrogen from the acid.
Example
Explain when Copper is added to dilute hydrochloric acid. No reaction occurs even on heating. Why?
▶️Answer/Explanation
Copper is below hydrogen in the reactivity series, so it cannot displace hydrogen from the acid. Thus, no bubbles are observed.
Displacement and positive ion formation
Displacement and positive ion formation
The reactivity of a metal is closely linked to how easily it loses electrons to form positive ions (cations).A more reactive metal loses electrons more readily, forming ions quickly. This principle can be demonstrated by displacement reactions between metals and the aqueous ions of other metals.
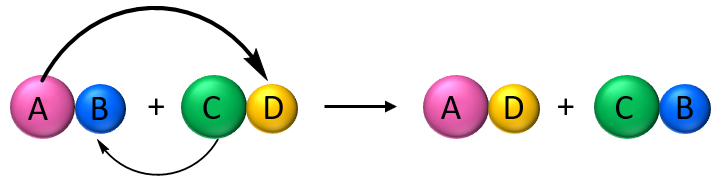
- If a more reactive metal is placed in a solution containing ions of a less reactive metal, the more reactive metal displaces the less reactive one. This is because the more reactive metal forms positive ions more readily, pushing the less reactive metal out of the solution.

General reaction:
Reactive metal + Metal salt solution → New salt solution + Less reactive metal
\( \text{A} + \text{B}^{2+}(aq) \rightarrow \text{A}^{2+}(aq) + \text{B} \)
(where A is more reactive than B)
Examples: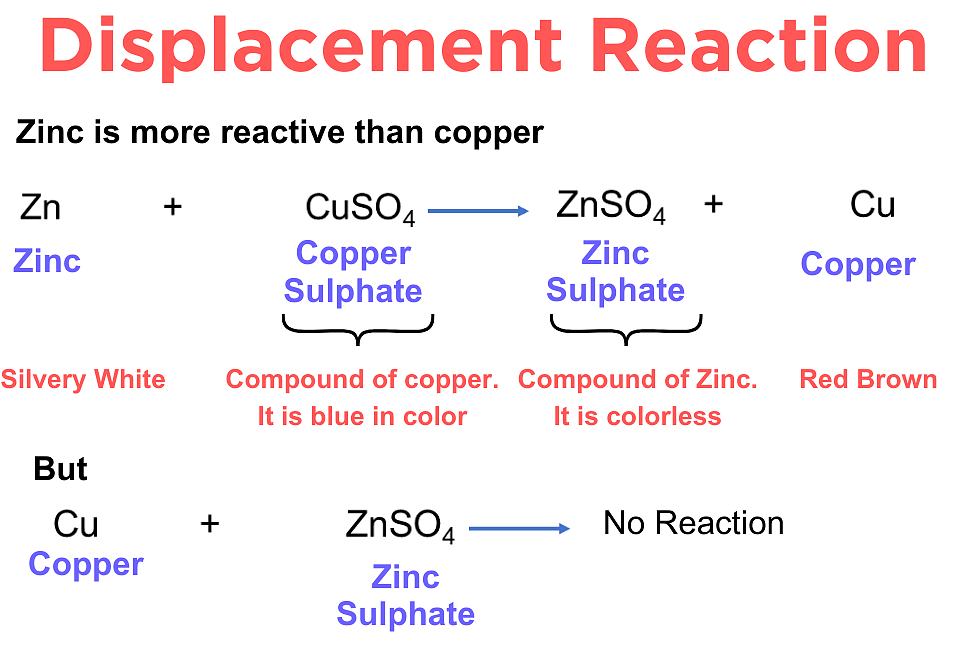
Zinc placed in copper(II) sulfate solution reacts. Zinc displaces copper because zinc is higher in the reactivity series. The solution changes from blue (copper sulfate) to colourless (zinc sulfate), and brown copper metal is deposited.
Equation: \( \text{Zn} + \text{CuSO}_4 \rightarrow \text{ZnSO}_4 + \text{Cu} \)
Iron placed in copper(II) sulfate solution reacts. Iron displaces copper, forming iron sulfate and copper metal.
Equation: \( \text{Fe} + \text{CuSO}_4 \rightarrow \text{FeSO}_4 + \text{Cu} \)
However, copper placed in zinc sulfate solution shows no reaction, because copper is less reactive than zinc and cannot displace it.
Key trends from displacement reactions
- Magnesium, zinc, and iron are able to displace copper and silver from their solutions, showing they are more reactive.
- Copper and silver cannot displace magnesium, zinc, or iron, showing they are less reactive.
- Gold is at the very bottom of the series, so it does not take part in displacement reactions at all.
Example
An iron nail is placed into silver nitrate solution. Write the reaction and explain it.
▶️Answer/Explanation
Reaction: \( \text{Fe (s)} + 2\text{AgNO}_3 (aq) \rightarrow \text{Fe(NO}_3\text{)}_2 (aq) + 2\text{Ag (s)} \)
Iron displaces silver because iron is more reactive than silver. Shiny silver crystals form on the iron nail, and the solution changes as iron(II) nitrate forms.
Example
A strip of copper is placed in magnesium sulfate solution. Explain
▶️Answer/Explanation
No reaction occurs because copper is less reactive than magnesium. Copper cannot displace magnesium from its solution.
Aluminium’s oxide layer
Aluminium’s oxide layer
Aluminium is a highly reactive metal and is placed above many other metals in the reactivity series. In theory, it should react rapidly with water and dilute acids, but in practice aluminium often appears to be unreactive. This apparent unreactivity is due to the presence of a thin but strong oxide layer on its surface.
Formation of the oxide layer: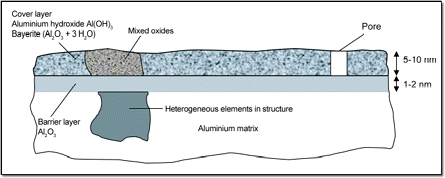
When aluminium is exposed to air, it reacts immediately with oxygen to form aluminium oxide, \( \text{Al}_2\text{O}_3 \). This oxide layer is very thin but tightly bonded to the surface of the metal. It forms almost instantly and prevents further oxygen or water from reaching the aluminium underneath.
Properties of the oxide layer:
The aluminium oxide layer is tough, hard, and impermeable. It acts as a protective barrier, stopping the aluminium metal beneath from reacting further. This explains why aluminium objects do not corrode easily and appear unreactive in water or dilute acids under normal conditions.
Consequences:
Aluminium does not seem to react with cold water, steam, or dilute acids because the oxide layer protects it. However, if the oxide layer is removed or disrupted (for example by scratching the surface, using powdered aluminium, or dissolving the oxide with strong alkalis), then aluminium reacts vigorously, showing its true reactivity.
For instance, powdered aluminium can react explosively with oxygen because the protective oxide layer is broken and a large surface area of pure aluminium is exposed.
Industrial importance:
The oxide layer makes aluminium useful in everyday life, since it prevents corrosion. Aluminium is therefore widely used for window frames, aircraft bodies, food packaging, and kitchen utensils. In industry, aluminium’s true high reactivity is harnessed in thermite reactions, where aluminium powder reduces iron(III) oxide to molten iron.
Example
Discuss the Use of aluminium in aircraft bodies.
▶️Answer/Explanation
Although aluminium is reactive, the strong oxide coating protects it from corrosion. This makes aluminium ideal for aircraft construction, where light weight and resistance to rusting are crucial.