Reversible reactions and equilibrium- CIE iGCSE Chemistry Notes - New Syllabus
Reversible reactions and equilibrium for iGCSE Chemistry Notes
Core Syllabus
- State that some chemical reactions are reversible as shown by the symbol ⇌
- Describe how changing the conditions can change the direction of a reversible reaction for:
(a) the effect of heat on hydrated compounds
(b) the addition of water to anhydrous compounds limited to copper(II) sulfate and cobalt(II) chloride
Supplement Syllabus
- State that a reversible reaction in a closed system is at equilibrium when:
(a) the rate of the forward reaction is equal to the rate of the reverse reaction
(b) the concentrations of reactants and products are no longer changing
Predict and explain, for a reversible reaction, how the position of equilibrium is affected by:
(a) changing temperature
(b) changing pressure
(c) changing concentration
(d) using a catalyst using information provided - State the symbol equation for the production of ammonia in the Haber process, $\text{N}_2(g) + 3\text{H}_2(g) ⇌ 2\text{NH}_3(g)$
- State the sources of the hydrogen (methane) and nitrogen (air) in the Haber process
- State the typical conditions in the Haber process as 450°C, 20000kPa/200atm and an iron catalyst
- State the symbol equation for the conversion of sulfur dioxide to sulfur trioxide in the Contact process, $2\text{SO}_2(g) + \text{O}_2(g) ⇌ 2\text{SO}_3(g)$
- State the sources of the sulfur dioxide (burning sulfur or roasting sulfide ores) and oxygen (air) in the Contact process
- State the typical conditions for the conversion of sulfur dioxide to sulfur trioxide in the Contact process as 450°C, 200kPa/2atm and a vanadium(V) oxide catalyst
- Explain, in terms of rate of reaction and position of equilibrium, why the typical conditions stated are used in the Haber process and in the Contact process, including safety considerations and economics
Reversible Reactions
Reversible Reactions
A reversible reaction is a chemical reaction in which the reactants form products, but those products can also react to reform the original reactants.
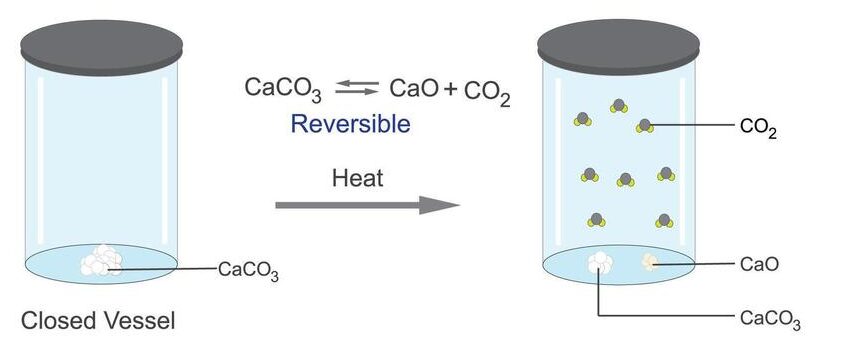
This means the reaction can proceed in both the forward and backward directions depending on the conditions.
The symbol used to indicate a reversible reaction is ⇌. This symbol shows that the reaction is not one-way, but that the products and reactants are constantly being interconverted.
What Makes a Reaction Reversible?
- The system must be in a closed container so that no substances escape.
- Both forward and reverse reactions can happen under the same conditions.
- Neither reactants nor products are used up completely.
Physical appearance may or may not change, but chemical composition remains in dynamic balance when equilibrium is reached.
Example
What happens in the reversible decomposition and recombination of ammonium chloride?
▶️Answer/Explanation
When solid ammonium chloride is heated, it decomposes into ammonia and hydrogen chloride gases:
\(\text{NH}_4\text{Cl}{(s)} \rightleftharpoons \text{NH}_3{(g)} + \text{HCl}_{(g)}\)
If the gases are cooled, they react and form back solid ammonium chloride. This shows a reversible reaction where products can form the reactant again.
Example
What does the symbol ⇌ indicate in the reaction between nitrogen and hydrogen forming ammonia?
▶️Answer/Explanation
The reaction: \( \text{N}_2{(g)} + 3\text{H}_2{(g)} \rightleftharpoons 2\text{NH}_3{(g)} \) shows that ammonia is formed from nitrogen and hydrogen (forward reaction), but under suitable conditions, ammonia can decompose back into nitrogen and hydrogen (reverse reaction).
This reversible process is used in the Haber process.
Effect of Changing Conditions on the Direction of Reversible Reactions
Changing the conditions of a reversible reaction can alter the direction in which the reaction proceeds. These changes can shift the equilibrium either towards the products (forward reaction) or towards the reactants (reverse reaction).
This point focuses on two key reversible reactions involving hydrated and anhydrous salts:
- Effect of heat on hydrated compounds
- Addition of water to anhydrous compounds
These reactions are usually physical changes and are easily reversible under appropriate conditions.
1. Hydrated Copper(II) Sulfate
When blue hydrated copper(II) sulfate is heated, it loses water and turns into white anhydrous copper(II) sulfate. When water is added again, the blue color returns.
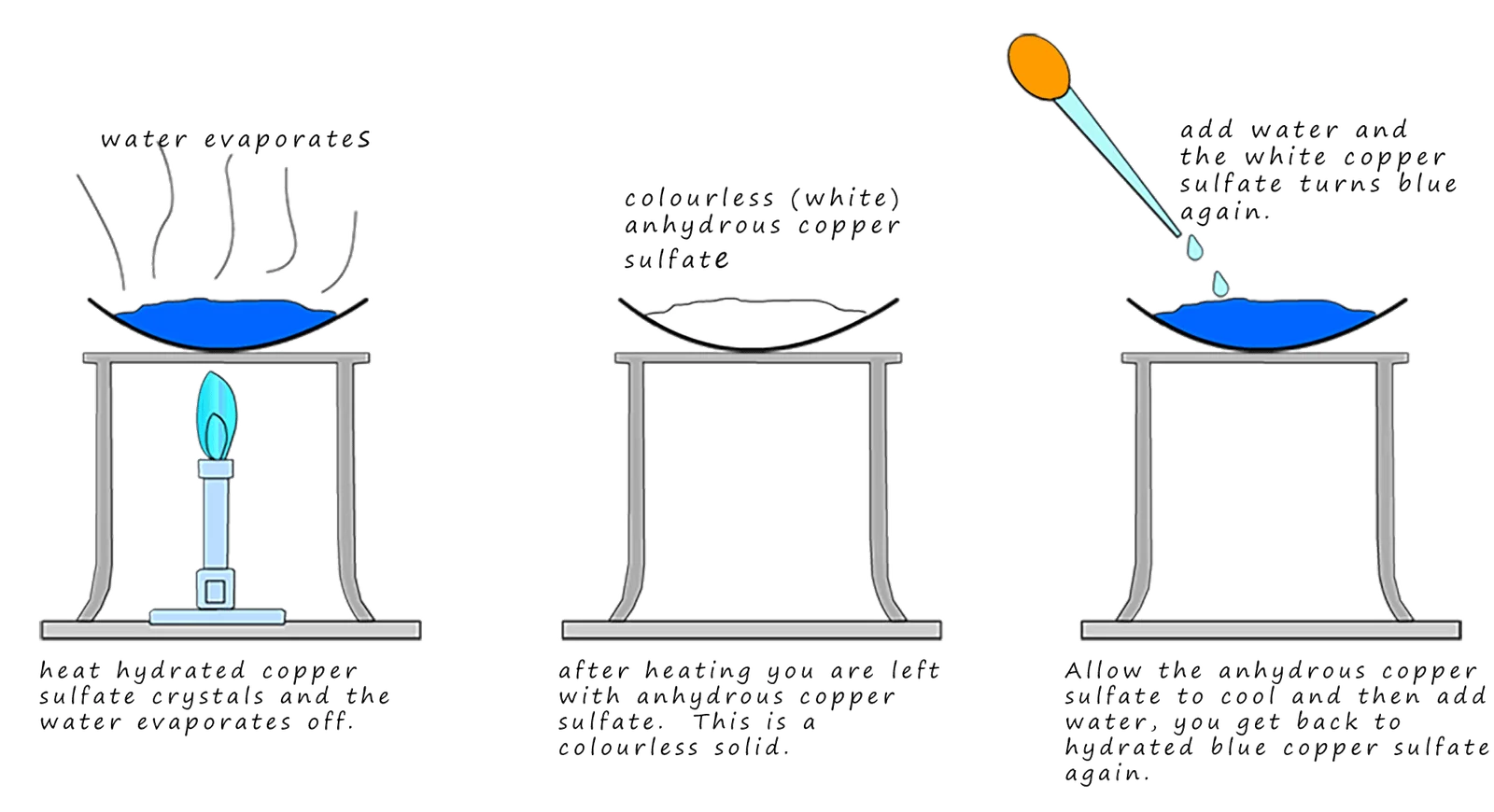
Reversible reaction:
\( \text{CuSO}_4\cdot5\text{H}_2\text{O}_{(s)} \rightleftharpoons \text{CuSO}_4{(s)} + 5\text{H}_2\text{O}{(l)} \)
Explanation:
- Heating: Drives the reaction in the forward direction. Water is lost, and the blue salt becomes white.
- Adding Water: Drives the reaction in the reverse direction. The white salt absorbs water and becomes blue again.
Example
What is observed when hydrated copper(II) sulfate is heated and then cooled with water?
▶️Answer/Explanation
Heating turns the blue hydrated salt white as water is driven off. On adding water again, the white powder turns back to blue. This is a clear example of a reversible reaction affected by heat and water.
2. Cobalt(II) Chloride
Cobalt(II) chloride also shows a color change when water is added or removed:
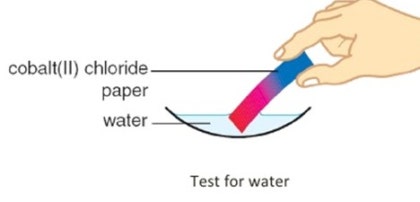
\( \text{CoCl}_2\cdot6\text{H}_2\text{O}{(s)} \rightleftharpoons \text{CoCl}_2{_{(s)}} + 6\text{H}_2\text{O}{_{(l)}} \)
- Hydrated cobalt(II) chloride is pink.
- Anhydrous cobalt(II) chloride is blue.
This reversible change is used in moisture indicator strips.
Example
How does cobalt(II) chloride demonstrate a reversible reaction when exposed to moist air?
▶️Answer/Explanation
In dry air, cobalt(II) chloride is blue (anhydrous). In moist air, it absorbs water and becomes pink (hydrated). Removing the moisture by heating returns the blue color. This shows the reaction is reversible and sensitive to water.
Dynamic Equilibrium
Dynamic Equilibrium
In a closed system, a reversible reaction can reach a state known as dynamic equilibrium.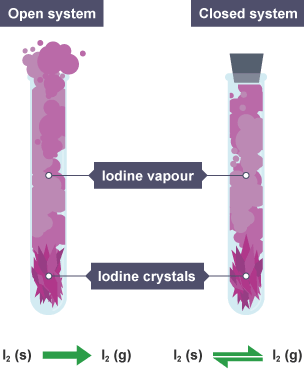
Dynamic equilibrium is the point at which:
- The rate of the forward reaction is equal to the rate of the reverse reaction.
- The concentrations of reactants and products remain constant over time, even though both reactions continue to occur.
Important conditions:
- The system must be closed – no substances are added or removed.
- Dynamic equilibrium does not mean that the amounts of reactants and products are equal – only that their concentrations are constant.
Explanation:
At equilibrium, both forward and reverse reactions are still occurring, but at the same rate. This makes the overall system appear static, but on the molecular level, it is dynamic. That is why we call it “dynamic equilibrium”.
Example
What does it mean for a reversible reaction to be at dynamic equilibrium?
▶️Answer/Explanation
It means that the rate of the forward reaction is equal to the rate of the reverse reaction, and the concentrations of all substances remain constant. For example, in the reaction:
\( \text{N}_2 + 3\text{H}_2 \rightleftharpoons 2\text{NH}_3 \)
when equilibrium is reached in a closed container, ammonia is still being formed and decomposed at the same rate, so the amount of each substance remains unchanged.
Effect of Changing Conditions on the Position of Equilibrium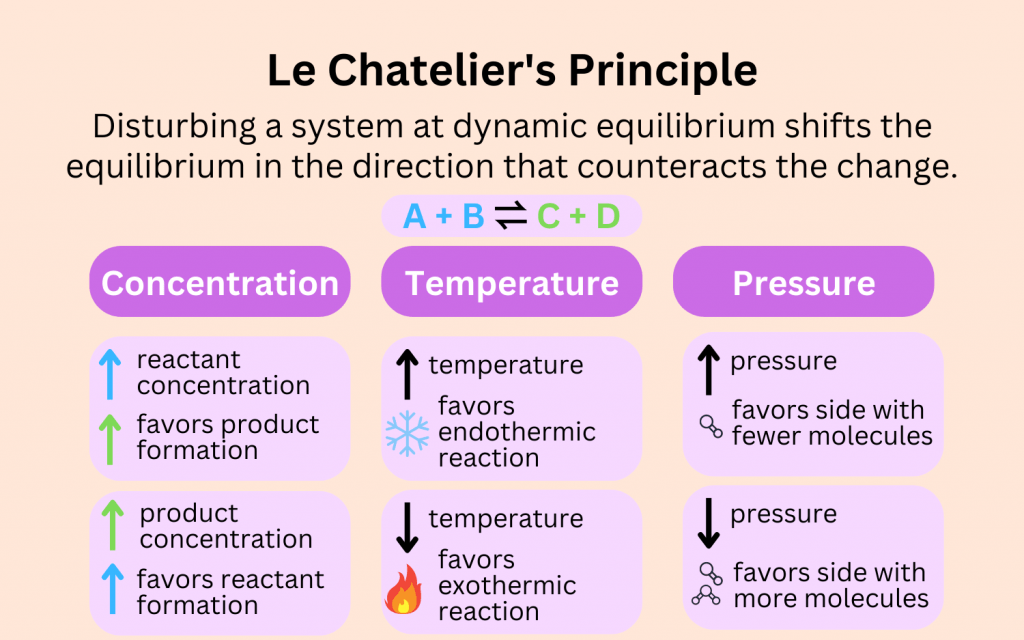
According to Le Chatelier’s Principle, if a system at equilibrium is disturbed by changing temperature, pressure, or concentration, the system will respond to oppose the change and restore a new equilibrium.
Let’s look at each condition:
- Temperature: Increasing temperature favors the endothermic direction. Decreasing temperature favors the exothermic direction.
- Pressure (for gases): Increasing pressure shifts equilibrium toward the side with fewer gas molecules. Decreasing pressure shifts it toward the side with more gas molecules.
- Concentration: Increasing the concentration of a reactant or product shifts the equilibrium to reduce that change (i.e., away from what was added).
- Catalyst: A catalyst speeds up both forward and reverse reactions equally. It does not change the position of equilibrium, only how fast it is reached.
Example
How does increasing the temperature affect the position of equilibrium in the reaction:
\( \text{N}_2(g) + 3\text{H}_2(g) \rightleftharpoons 2\text{NH}_3(g) \) (ΔH = -92 kJ/mol)
▶️Answer/Explanation
This reaction is exothermic. Increasing temperature shifts the equilibrium to the left (reverse direction) to absorb the added heat. As a result, less ammonia is formed.
Example
What happens when pressure is increased in the reaction:
\( \text{N}_2(g) + 3\text{H}_2(g) \rightleftharpoons 2\text{NH}_3(g) \)
▶️Answer/Explanation
On the left, there are 4 gas molecules (1 N₂ + 3 H₂), and on the right, there are 2 NH₃ molecules. Increasing pressure shifts the equilibrium to the right (toward fewer molecules), increasing ammonia yield.
Example
Does adding a catalyst to a reversible reaction affect the position of equilibrium?
▶️Answer/Explanation
No, a catalyst does not change the position of equilibrium. It increases the rate of both the forward and reverse reactions equally, allowing equilibrium to be reached faster.
Haber Process: Symbol Equation and Key Information
Haber Process: Symbol Equation and Key Information
The Haber Process is a key industrial method used to produce ammonia, a compound vital for the manufacture of fertilizers, explosives, and cleaning products.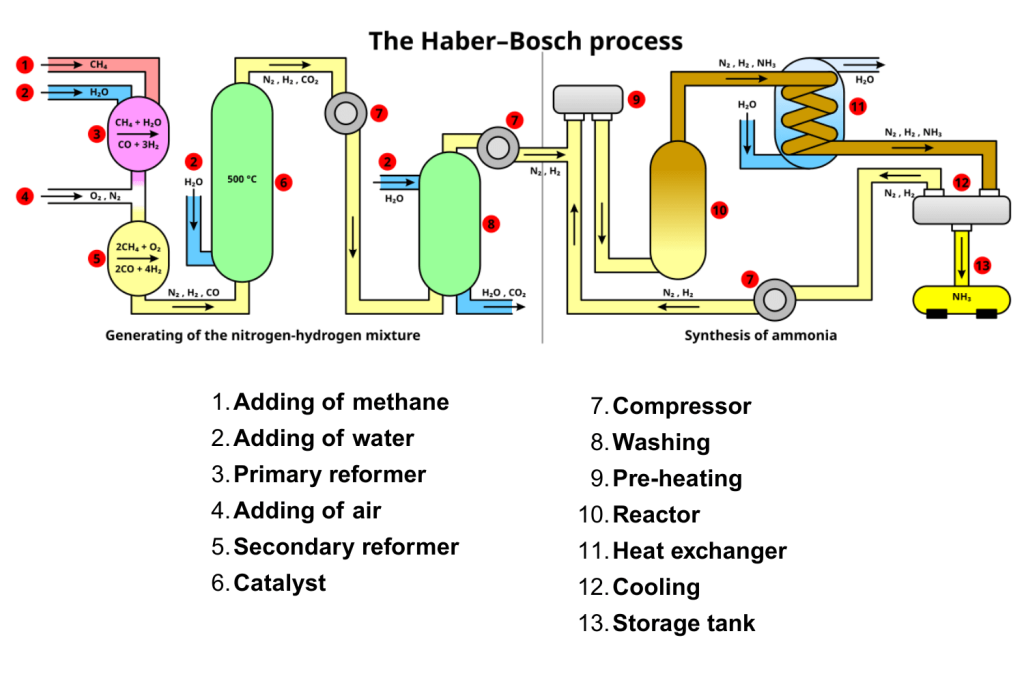
The balanced symbol equation for the Haber Process is:
\( \text{N}_2{(g)} + 3\text{H}_2{(g)} \rightleftharpoons 2\text{NH}_3{(g)} \)
This is a reversible reaction that occurs under specific conditions and reaches a dynamic equilibrium in a closed system.
Key Characteristics of the Equation:
- One molecule of nitrogen reacts with three molecules of hydrogen.
- Two molecules of ammonia are produced.
- The symbol \( \rightleftharpoons \) shows that the reaction is reversible.
Important Notes:
- Nitrogen is obtained from the air, which is about 78% nitrogen by volume.
- Hydrogen is usually obtained by reacting methane (from natural gas) with steam.
- The reaction is exothermic — it releases heat.
Example
Write a balanced symbol equation for the reaction in the Haber Process and identify the physical states of all the reactants and products.
▶️Answer/Explanation
The balanced equation is:
\( \text{N}_2{(g)} + 3\text{H}_2{(g)} \rightleftharpoons 2\text{NH}_3{(g)} \)
All substances are gases:
Nitrogen and hydrogen react to form ammonia.
The double arrow indicates that this is a reversible reaction.
Sources of Reactants in the Haber Process
In the Haber Process, the two key reactants are hydrogen and nitrogen. These are sourced from abundant and inexpensive raw materials:
Source of Nitrogen: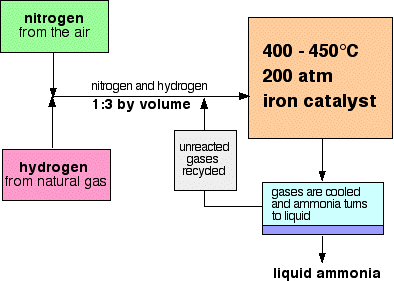
- Nitrogen is obtained directly from the air, which contains approximately 78% nitrogen by volume.
- The air is first cooled and compressed, then the nitrogen is separated by fractional distillation.
Source of Hydrogen:
- Hydrogen is obtained by reacting methane (from natural gas) with steam in a process called steam reforming.
- The reaction is: \( \text{CH}_4 + \text{H}_2\text{O} \rightarrow \text{CO} + 3\text{H}_2 \)
- This process is carried out at high temperatures using a nickel catalyst.
Summary:
- Nitrogen source — air (separated by fractional distillation).
- Hydrogen source — natural gas (methane), reacted with steam.
Example
Identify the sources of hydrogen and nitrogen for use in the Haber Process. Write the reaction used to produce hydrogen.
▶️Answer/Explanation
Sources:
– Nitrogen is obtained from air (78%).
– Hydrogen is obtained by reacting methane with steam:
\( \text{CH}_4 + \text{H}_2\text{O} \rightarrow \text{CO} + 3\text{H}_2 \)
This reaction requires high temperature and a nickel catalyst.
Typical Conditions in the Haber Process
The Haber Process is governed by both kinetic (rate of reaction) and thermodynamic (equilibrium position) factors. The conditions chosen — 450 °C, 200 atm, and an iron catalyst — are a result of compromises between yield, speed, safety, and cost.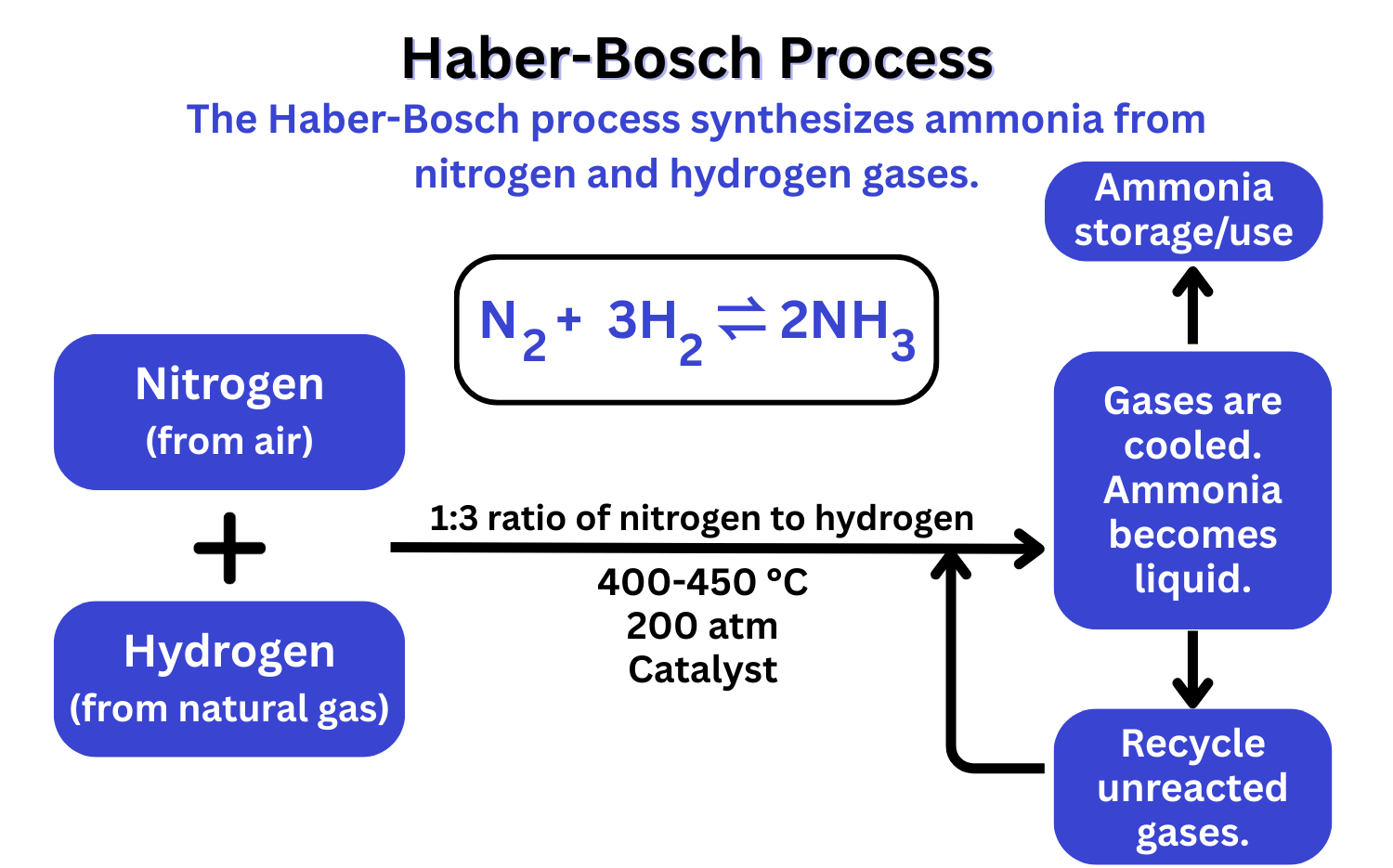
1. Temperature (450 °C):
Since the reaction is exothermic, lowering temperature favours the forward reaction and increases ammonia yield. However, too low a temperature slows down the reaction rate significantly. So, 450 °C is chosen as a compromise: it allows a reasonable rate of ammonia production while still giving an acceptable yield.
2. Pressure (200 atm):
High pressure shifts the equilibrium towards ammonia because the number of moles of gas decreases (4 reactant moles → 2 product moles). But extremely high pressure would require thick, strong reaction vessels — increasing cost and risk. A pressure of 200 atm provides increased yield while being economically feasible and safe enough.
3. Catalyst (Iron):
The iron catalyst does not shift the equilibrium but increases the rate of both forward and reverse reactions. This helps the system reach equilibrium faster. It also lowers the energy needed, making the process more efficient. Iron is inexpensive and widely available, making it ideal for industrial use.
4. Safety Considerations:
- Extremely high pressures are dangerous — risk of explosion or rupture in equipment.
- Very low temperatures may cause operational inefficiency and longer processing times.
5. Economic Considerations:
- Energy costs rise with higher pressure and temperature — so moderate values are selected.
- The equipment needed for extremely high pressures is expensive.
- The use of an iron catalyst reduces the energy cost and increases productivity.
Example
Explain why the following conditions are used in the Haber Process: 450 °C temperature, 200 atm pressure, and an iron catalyst. Include considerations of equilibrium, rate, safety, and economics.
▶️Answer/Explanation
Temperature: 450 °C is a compromise between higher yield at lower temperatures and faster reaction at higher temperatures.
Pressure: 200 atm favours ammonia production while being cost-effective and safe.
Catalyst: Iron speeds up the reaction without affecting yield, helping reach equilibrium faster.
Safety: Very high pressures are risky; controlled conditions are safer.
Economics: Equipment and energy costs are minimised by using moderate pressure and a catalyst.
Symbol Equation for the Contact Process
Symbol Equation for the Contact Process
The Contact Process is used to manufacture sulfuric acid. The key step in this process is the conversion of sulfur dioxide (\( \text{SO}_2 \)) into sulfur trioxide (\( \text{SO}_3 \)) using oxygen. This reaction is reversible and occurs in the presence of a catalyst.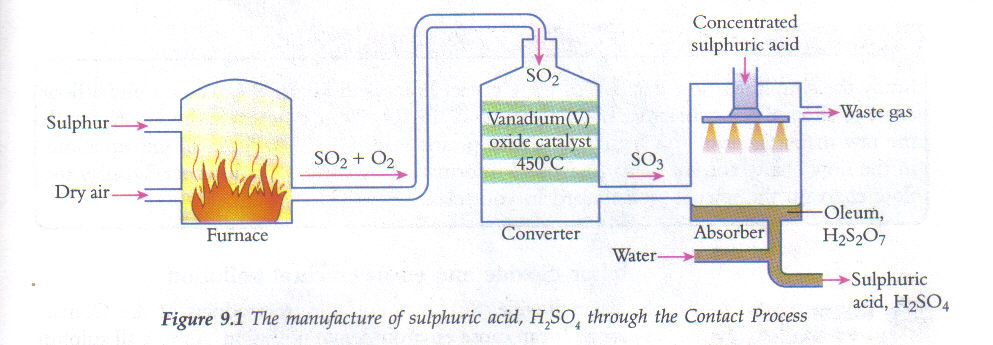
Balanced Symbol Equation:
\( 2\text{SO}_2(g) + \text{O}_2(g) \ ⇌\ 2\text{SO}_3(g) \)
Key Features:
- This is a reversible reaction, indicated by the symbol \( \ ⇌ \).
- It is an example of an equilibrium reaction carried out under controlled conditions.
- It is catalysed by vanadium(V) oxide (\( \text{V}_2\text{O}_5 \)).
- The forward reaction is exothermic, releasing heat.
Example
Write the balanced symbol equation for the key reaction in the Contact Process used to produce sulfur trioxide from sulfur dioxide and oxygen.
▶️Answer/Explanation
The key reaction in the Contact Process is:
\( 2\text{SO}_2(g) + \text{O}_2(g) \ ⇌\ 2\text{SO}_3(g) \)
This reaction occurs in the presence of a vanadium(V) oxide catalyst and is exothermic in the forward direction. The reaction reaches dynamic equilibrium under suitable conditions of temperature and pressure.
Sources of Reactants in the Contact Process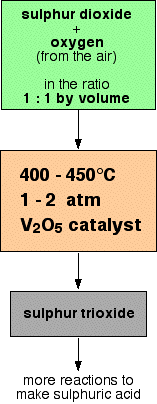
The Contact Process involves the oxidation of sulfur dioxide to sulfur trioxide, which is then used to produce sulfuric acid. To carry out this process, large-scale industrial plants require reliable sources of both sulfur dioxide and oxygen.
Sources of Sulfur Dioxide (\( \text{SO}_2 \)):
- Burning elemental sulfur in air:
\( \text{S}(s) + \text{O}_2(g) \rightarrow \text{SO}_2(g) \) - Roasting metal sulfide ores such as zinc blende (\( \text{ZnS} \)) or iron pyrite (\( \text{FeS}_2 \)):
\( 2\text{ZnS}(s) + 3\text{O}_2(g) \rightarrow 2\text{ZnO}(s) + 2\text{SO}_2(g) \)
\( 4\text{FeS}_2(s) + 11\text{O}_2(g) \rightarrow 2\text{Fe}_2\text{O}_3(s) + 8\text{SO}_2(g) \)
Source of Oxygen (\( \text{O}_2 \)):
- Oxygen is obtained from the air, which contains approximately 21% oxygen by volume.
Example
Identify the industrial sources of sulfur dioxide and oxygen used in the Contact Process.
▶️Answer/Explanation
Sulfur dioxide (\( \text{SO}_2 \)) is produced by either burning elemental sulfur in air or roasting sulfide ores such as zinc sulfide (\( \text{ZnS} \)) and iron pyrite (\( \text{FeS}_2 \)).
Oxygen (\( \text{O}_2 \)) is simply obtained from the air, which is readily available and free of cost.
Example
What are the typical conditions used in the industrial conversion of sulfur dioxide to sulfur trioxide in the Contact Process? Explain why each condition is chosen.
▶️Answer/Explanation
Temperature: 450 °C — high enough to give a fast reaction rate while maintaining a reasonable yield.
Pressure: 200 kPa — a low to moderate pressure that is economical and safer while still offering acceptable yield.
Catalyst: Vanadium(V) oxide (\( \text{V}_2\text{O}_5 \)) — used to increase the rate of reaction without affecting the equilibrium position.
Why Typical Conditions Are Used in the Contact Process
The Contact Process converts sulfur dioxide to sulfur trioxide by the reversible reaction:
\( 2\text{SO}_2(g) + \text{O}_2(g) \rightleftharpoons 2\text{SO}_3(g) \)
This reaction is:
- Exothermic — releases heat
- Involves a decrease in the number of gas molecules (from 3 to 2)
Effect of Temperature: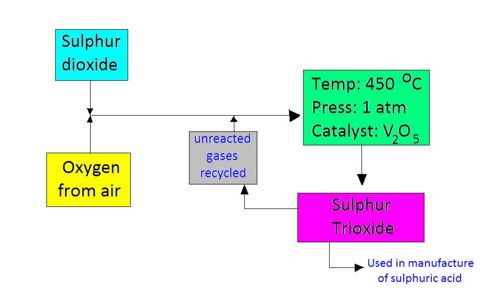
- Lower temperatures would favor the forward reaction (due to it being exothermic) and increase yield of \( \text{SO}_3 \).
- However, lower temperatures reduce the rate of reaction, making the process inefficient.
- Compromise temperature of 450 °C is chosen — it provides a reasonable yield with a fast enough rate.
Effect of Pressure:
- Higher pressures favor the forward reaction because it reduces the number of gas molecules (from 3 to 2).
- However, the yield increase is small, and high pressures are costly and pose safety risks.
- Moderate pressure of 200 kPa (2 atm) is used — it balances yield, cost, and safety.
Effect of Catalyst:
- Vanadium(V) oxide (\( \text{V}_2\text{O}_5 \)) is used as a catalyst.
- It increases the rate of reaction without affecting the equilibrium position.
- Enables operation at lower temperatures, improving efficiency.
Economic and Safety Considerations:
- Operating at lower temperatures saves energy and reduces costs.
- Using moderate pressure avoids the need for expensive equipment and lowers explosion risk.
- A catalyst allows fast production rates without extreme conditions.
Example
Explain why a temperature of 450 °C and a pressure of 200 kPa are used in the Contact Process, instead of higher pressure and lower temperature.
▶️Answer/Explanation
A lower temperature would increase the yield of \( \text{SO}_3 \) due to the exothermic forward reaction, but it would slow the rate of reaction too much. A temperature of 450 °C provides a good compromise.
A higher pressure would increase the yield of \( \text{SO}_3 \), but only slightly, and the cost and safety risks would outweigh the benefits. Therefore, a moderate pressure of 200 kPa is chosen.
The vanadium(V) oxide catalyst ensures that the rate is still fast even at this moderate temperature, reducing costs while maintaining good productivity.
