Separation and purification- CIE iGCSE Chemistry Notes - New Syllabus
Separation and purification for iGCSE Chemistry Notes
Core Syllabus
- Describe and explain methods of separation and purification using:
(a) a suitable solvent
(b) filtration
(c) crystallisation
(d) simple distillation
(e) fractional distillation - Suggest suitable separation and purification techniques, given information about the substances involved
- Identify substances and assess their purity using melting point and boiling point information
Methods of separation and purification
Methods of separation and purification
(a) Using a suitable solvent
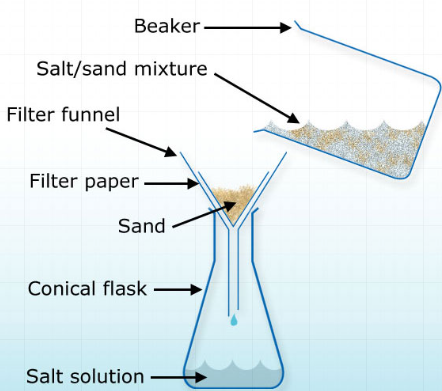
- A solvent is chosen that dissolves one substance in a mixture but not the other
- Example: A mixture of salt and sand → water dissolves salt but not sand → filtration separates the dissolved salt solution from sand
- Explanation: Different solubilities in the solvent allow selective separation
(b) Filtration
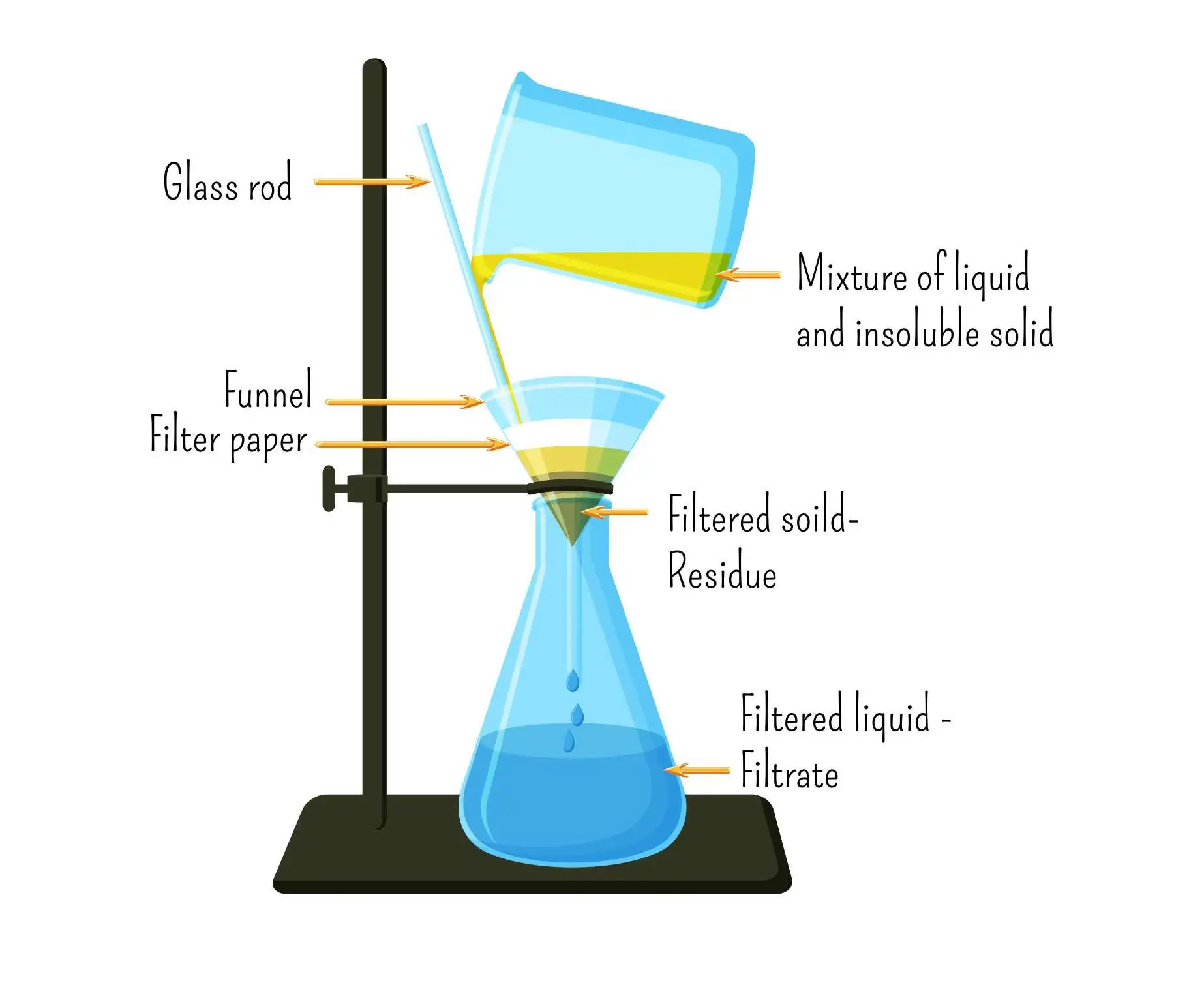
- Used to separate an insoluble solid from a liquid
- The mixture is poured through filter paper in a funnel
- The liquid (filtrate) passes through, while the insoluble solid (residue) remains on the paper
- Example: Separating sand from water
(c) Crystallisation
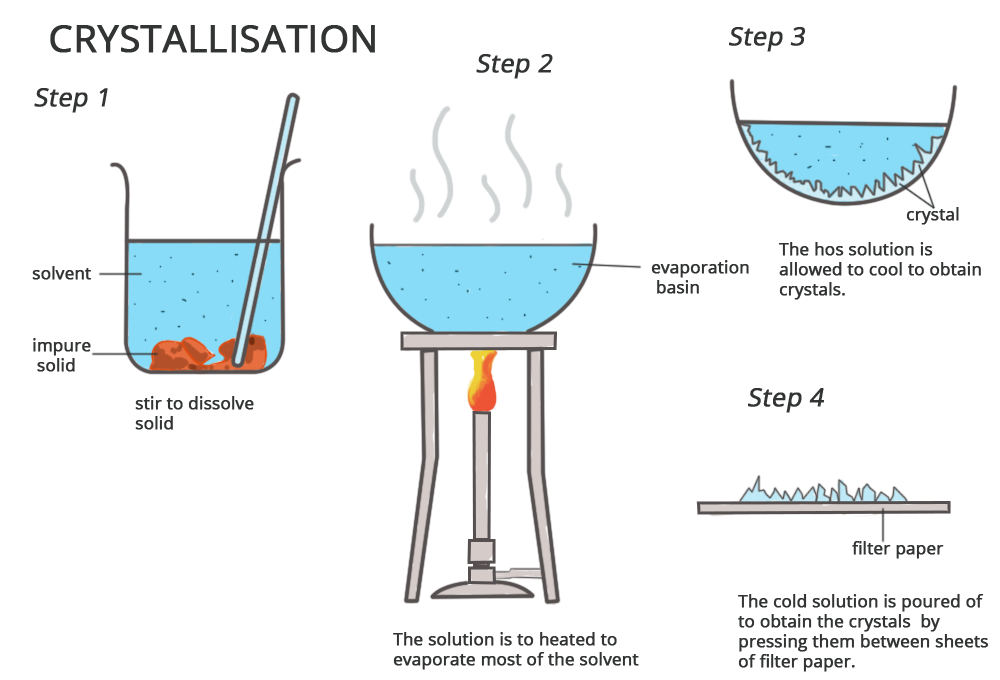
- Used to obtain a pure solid from a solution
- The solution is heated to evaporate part of the solvent, making it more concentrated
- Then the hot solution is cooled so crystals form
- Crystals are collected by filtration and dried
- Example: Producing pure copper(II) sulfate crystals from a solution
(d) Simple distillation
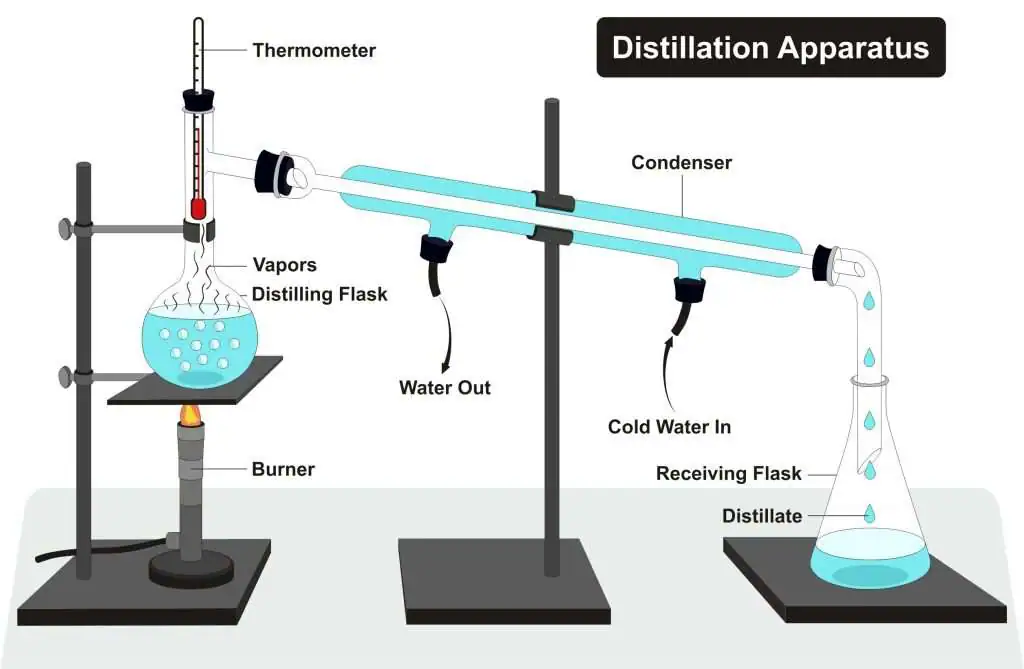
- Used to separate a liquid from a solution
- The solution is heated until the liquid boils
- The vapor rises, passes into a condenser, and condenses back into pure liquid, which is collected
- The dissolved solid remains in the flask
- Example: Obtaining pure water from salt water
(e) Fractional distillation
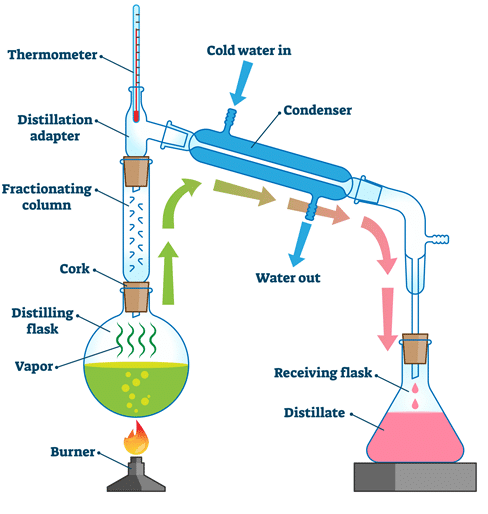
- Used to separate a mixture of liquids with different boiling points
- The mixture is heated, and the liquid with the lowest boiling point evaporates first
- The vapors pass through a fractionating column, which helps separate components
- The vapors condense in the condenser and are collected
- By gradually raising the temperature, different fractions are collected in order of increasing boiling points
- Example: Separating ethanol from water, or separating crude oil into fractions like petrol and diesel
Example
A mixture contains ethanol (boiling point 78 °C) and water (boiling point 100 °C). Suggest a method to separate them.
▶️Answer/Explanation
Fractional distillation should be used.
When the mixture is heated, ethanol boils and evaporates first at 78 °C. The vapor passes into the condenser and is collected as pure ethanol. Water remains in the flask until the temperature reaches 100 °C.
Suggest suitable separation and purification techniques, given information about the substances involved
To choose the correct separation technique, we must consider the physical properties of the substances involved (such as solubility, boiling point, or state). Below are common scenarios with the suitable method:
- Insoluble solid + liquid: Use filtration
Example: Sand and water
- Soluble solid + liquid: Use crystallisation (to obtain the solid) or simple distillation (to obtain the solvent)
Example: Salt solution → crystallisation gives pure salt; distillation gives pure water
- Two miscible liquids with different boiling points: Use fractional distillation
Example: Ethanol and water
- Two immiscible liquids (do not mix, form separate layers): Use a separating funnel
Example: Oil and water
- Soluble coloured substances: Use paper chromatography
Example: Dyes in inks
- Gas collection: Use gas syringe or an upturned measuring cylinder depending on solubility in water
Example: Oxygen (insoluble) collected over water; Carbon dioxide (more soluble) better with gas syringe
Example
A mixture contains sand, salt, and water. Suggest how to separate and obtain pure samples of each component.
▶️Answer/Explanation
Step 1: Filter the mixture → sand (residue) is separated from salt solution (filtrate).
Step 2: Evaporate or crystallise the salt solution → pure salt crystals remain.
Step 3: Collect the evaporated water separately using simple distillation → pure water is obtained.
Identifying substances and assessing their purity
Identifying substances and assessing their purity
One of the most reliable ways to determine whether a substance is pure or impure is by comparing its melting point or boiling point with the known values for the pure substance.
Pure substances
- They have sharp, fixed melting and boiling points.
Example:
- Pure water boils at 100°C at 1 atm pressure and pure ice melts at exactly 0°C. Any small deviation from these values would indicate the presence of impurities.
- The sharpness of these values is because all the particles are identical, so they require the same amount of energy to overcome the forces holding them together.
Impure substances
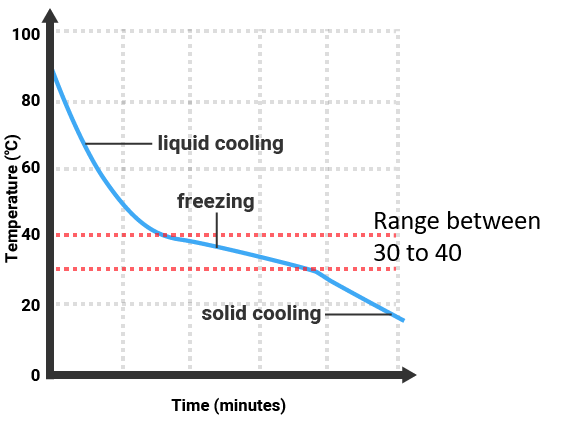
- They do not melt or boil at a single sharp temperature. Instead:
- Impure solids melt over a range of temperatures, usually lower than the pure melting point.
- Impure liquids boil over a range of temperatures, usually higher than the pure boiling point.
- This happens because impurities disrupt the regular arrangement of particles in solids and alter the strength of intermolecular forces in liquids, requiring different amounts of energy for different parts of the sample.
Effect of impurities
- Melting point depression: impurities lower the melting point and broaden the melting range.
- Boiling point elevation: impurities raise the boiling point and broaden the boiling range.
This principle is widely used in chemistry to check for purity.
Practical applications
- Melting point determination is commonly used in organic chemistry to test the purity of crystalline substances such as benzoic acid or aspirin.
- Boiling point determination is often used for checking the purity of liquids such as ethanol or distilled water.
- In industry and laboratories, purity checks are essential because even small impurities can change the properties and usefulness of substances.
Example
A student measures the melting point of a sample of benzoic acid. The substance melts gradually between 118°C and 121°C. The data book states that pure benzoic acid melts sharply at 122°C. What conclusion can be made about the sample?
▶️Answer/Explanation
The substance does not melt sharply at 122°C but instead melts over a range of temperatures that is slightly lower. This shows that the benzoic acid sample is impure. The impurities present disrupt the crystal lattice, lowering the melting point and causing the range instead of a sharp value.
