Simple molecules and covalent bonds- CIE iGCSE Chemistry Notes - New Syllabus
Simple molecules and covalent bonds for iGCSE Chemistry Notes
Core Syllabus
- State that a covalent bond is formed when a pair of electrons is shared between two atoms leading to noble gas electronic configurations
- Describe the formation of covalent bonds in simple molecules, including H₂, Cl₂, H₂O, CH₄, NH₃ and HCl. Use dot-and-cross diagrams to show the electronic configurations in these and similar molecules
- Describe in terms of structure and bonding the properties of simple molecular compounds:
(a) low melting points and boiling points
(b) poor electrical conductivity
Supplement Syllabus
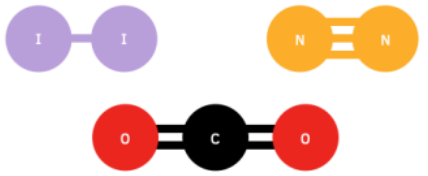
- Describe the formation of covalent bonds in simple molecules, including CH₃OH, C₂H₄, O₂, CO₂ and N₂. Use dot-and-cross diagrams to show the electronic configurations in these and similar molecules
- Explain in terms of structure and bonding the properties of simple molecular compounds:
(a) low melting points and boiling points in terms of weak intermolecular forces (specific types of intermolecular forces are not required)
(b) poor electrical conductivity
Covalent Bond
Covalent Bond
A covalent bond is a chemical bond formed when a pair of electrons is shared between two non-metal atoms. Each atom contributes one electron to the shared pair, creating a stable bond.
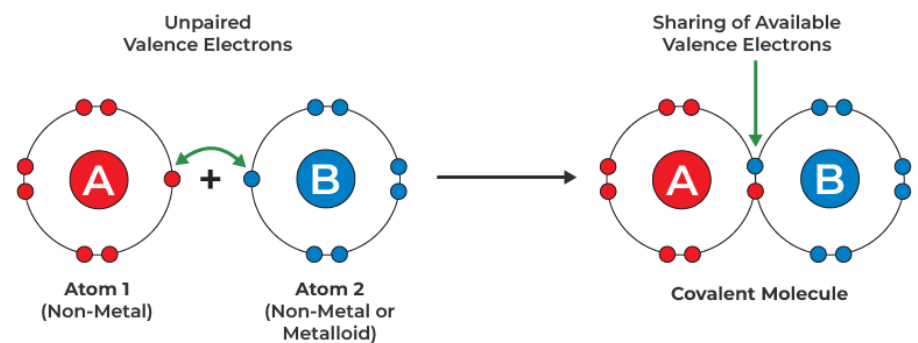
Purpose of Covalent Bonding:
The main reason atoms form covalent bonds is to achieve a full outer shell of electrons, which gives them the same electronic configuration as a noble gas. This makes the atoms more stable. For example:
- Hydrogen (H): Has 1 electron. Needs 1 more to achieve 2 electrons like Helium (He).
- Chlorine (Cl): Has 7 electrons in its outer shell. Needs 1 more to reach 8 like Argon (Ar).
- Oxygen (O): Has 6 outer electrons. Needs 2 more to reach 8.
Examples:
- Hydrogen molecule (H2): Each hydrogen shares 1 electron with another, giving both atoms 2 electrons in their outer shell.
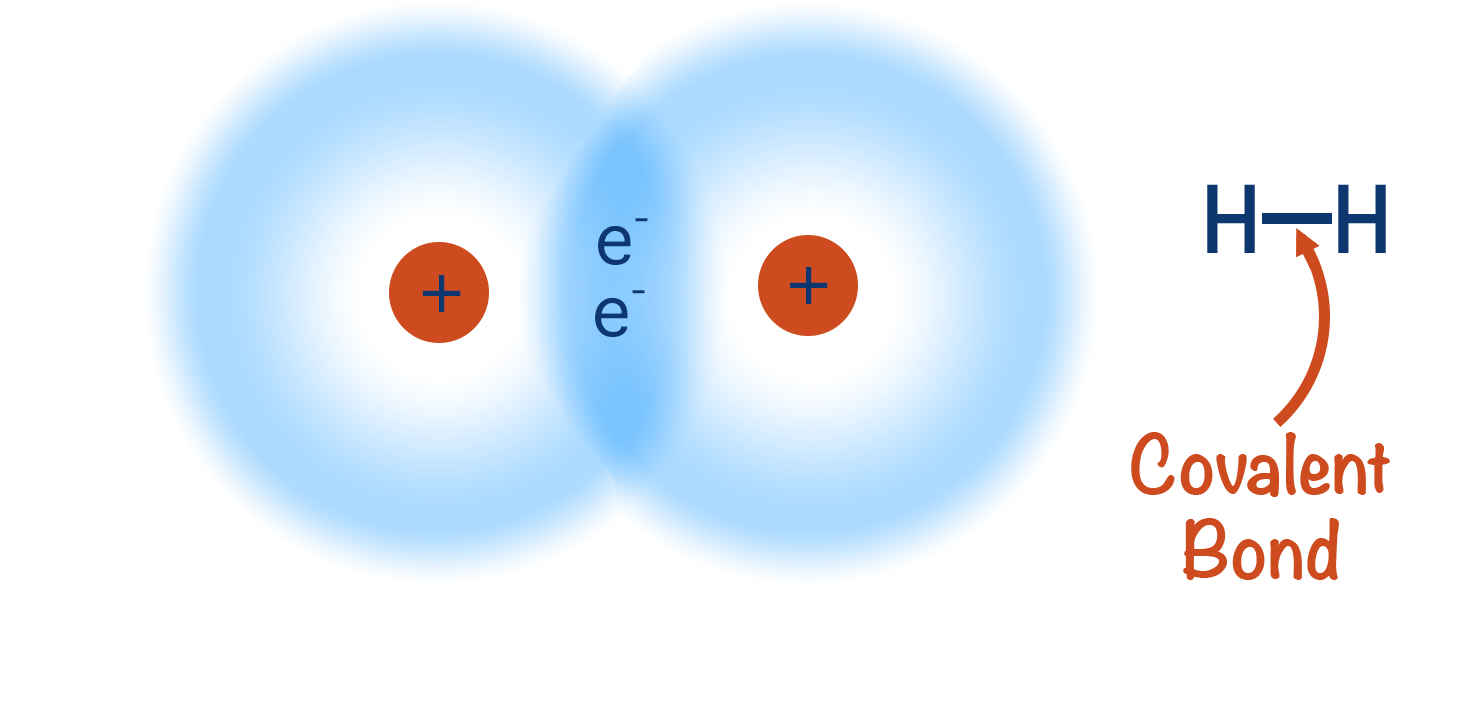
- Chlorine molecule (Cl2): Each chlorine shares 1 electron with another chlorine atom, giving both 8 electrons in the outer shell.
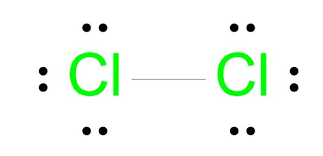
General Characteristics of Covalent Bonds:
- Formed between non-metallic elements.
- Involve the sharing (not transfer) of electrons.
- Each shared pair of electrons creates one covalent bond.
- Can involve single, double or triple bonds depending on how many electron pairs are shared.
- Atoms involved attain the noble gas configuration.
- The resulting structure is usually a simple molecule with a definite number of atoms covalently bonded together.
Key Difference from Ionic Bonding:
In covalent bonding, electrons are shared between atoms. In contrast, in ionic bonding, electrons are transferred from a metal to a non-metal, creating ions.
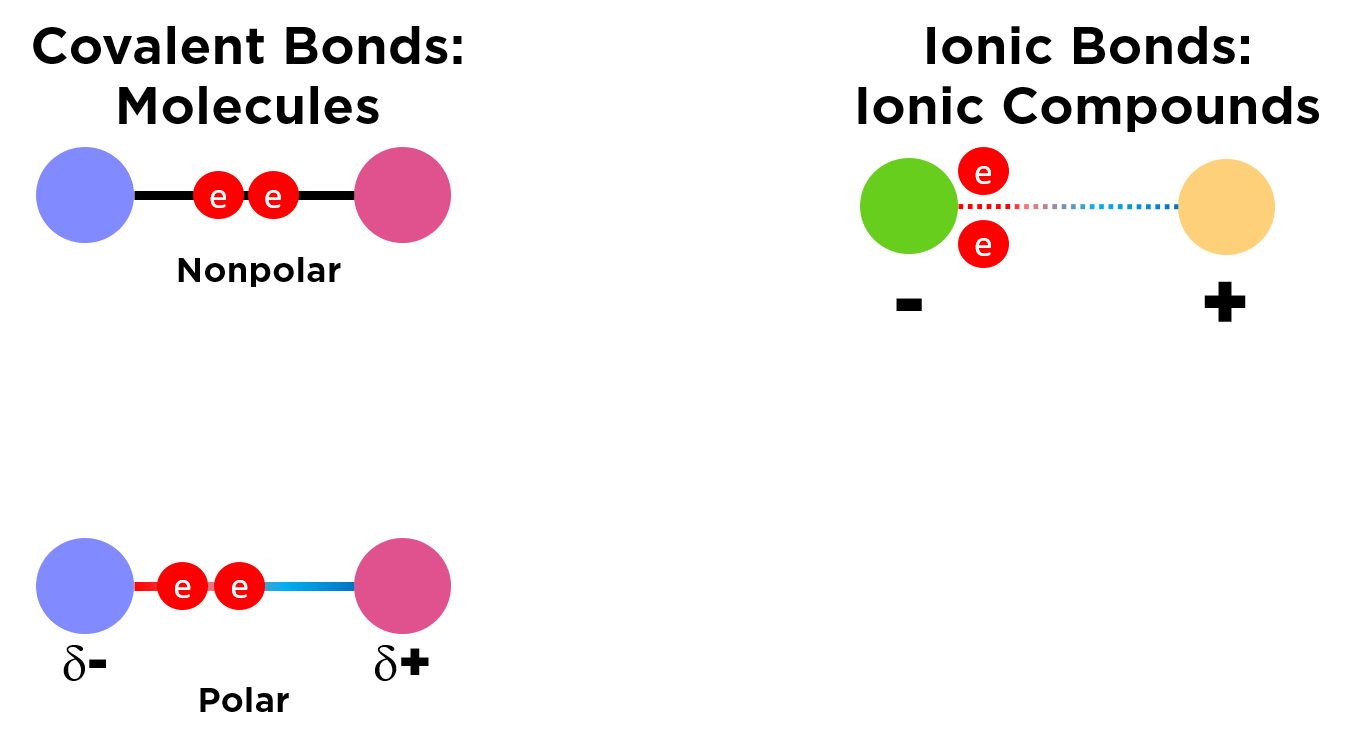
Formation of Covalent Bonds in Simple Molecules
Covalent bonds are formed when atoms share pairs of electrons to achieve a full outer shell (noble gas configuration). These bonds are typically shown using dot-and-cross diagrams, where:
- Dots represent electrons from one atom.
- Crosses represent electrons from the other atom.
- Only outer shell electrons are shown in these diagrams.
The number of bonds formed by an atom usually depends on how many electrons it needs to complete its outer shell:
- Hydrogen (H): 1 bond
- Oxygen (O): 2 bonds
- Nitrogen (N): 3 bonds
- Carbon (C): 4 bonds
- Halogens (Cl, Br, etc.): 1 bond
| Molecule | Atoms Involved | Type of Bonds | No. of Covalent Bonds | Special Notes | Dot-and-Cross Diagram |
|---|---|---|---|---|---|
| H2 | H – H | Single | 1 | Each hydrogen shares 1 electron | 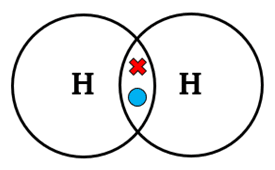 |
| Cl2 | Cl – Cl | Single | 1 | Each chlorine shares 1 electron to complete its octet | 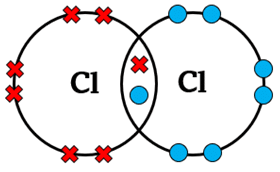 |
| H2O | H – O – H | Single | 2 | Oxygen shares 2 electrons with 2 hydrogen atoms | 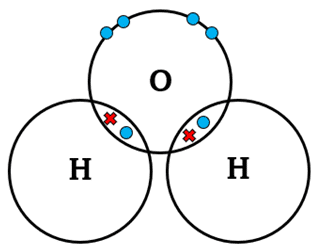 |
| CH4 | H – C – H (4 Hs) | Single | 4 | Carbon shares 1 electron with each hydrogen | 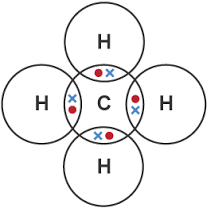 |
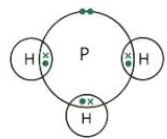
| NH3 | H – N – H (3 Hs) | Single | 3 | Nitrogen forms 3 covalent bonds with 3 hydrogen atoms | 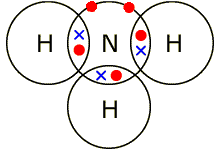 |
| HCl | H – Cl | Single | 1 | H shares 1 electron with Cl | 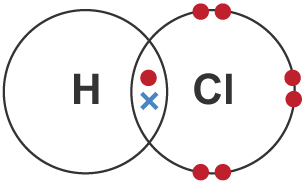 |
| CH3OH (methanol) | C, H, O | Single | 6 total (4 C-H, 1 C-O, 1 O-H) | Carbon bonds to 3 H + 1 O; O bonds to 1 H | 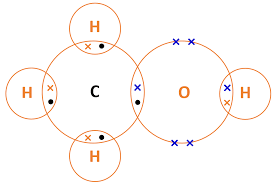 |
| C2H4 (ethene) | C = C and 4 H | Double (C=C), Single (C-H) | 5 total (1 double, 4 single) | Each C shares 2 electrons with the other + bonds to 2 H | 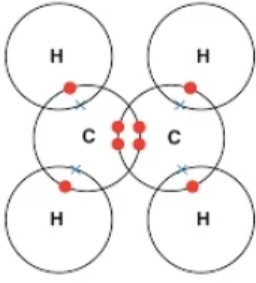 |
| O2 | O = O | Double | 1 (double) | Each oxygen shares 2 electrons with the other | 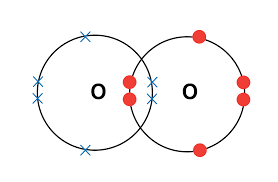 |
| CO2 | O = C = O | Two double bonds | 2 (double) | Carbon forms double bonds with two oxygen atoms | 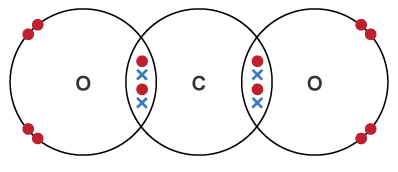 |
| N2 | N ≡ N | Triple | 1 (triple) | Each nitrogen shares 3 electrons with the other | 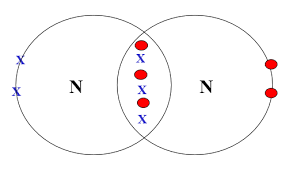 |
Example:
Each oxygen atom forms a single covalent bond with the other oxygen and one with a hydrogen in Hydrogen Peroxide (H2O2). Give dot and cross structure.
▶️Answer/Explanation
Both oxygen atoms obey the octet rule, and hydrogen atoms have 2 electrons each.
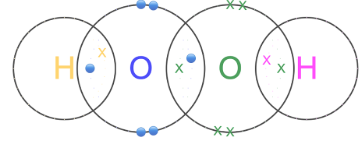
Example:
Phosphorus shares 1 electron with each of 3 hydrogen atoms, similar to NH3 in Phosphine (PH3). Give dot and cross structure.
▶️Answer/Explanation
P has 5 outer electrons and shares 3 to complete its octet.
Properties of Simple Molecular Substances
Properties of Simple Molecular Substances
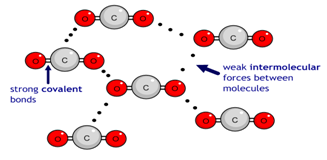
Simple molecular substances are made up of discrete molecules held together by strong covalent bonds within the molecule and weak intermolecular forces between the molecules. These include compounds like water (H2O), carbon dioxide (CO2), ammonia (NH3), and hydrogen (H2).
1. Melting and Boiling Points
Simple molecular substances generally have low melting and boiling points.
- The covalent bonds inside the molecules are very strong and do not break during melting or boiling.
- However, the intermolecular forces (forces between molecules) are weak and require only a small amount of energy to overcome. The weak intermolecular forces (such as van der Waals forces or dipole-dipole interactions) require little energy to break, even though the covalent bonds within each molecule are strong. Since the molecules are easily separated from one another, the melting and boiling points are low.
- So, these substances usually exist as gases or liquids at room temperature, or as soft solids with low melting points.
2. Electrical Conductivity
Simple molecular substances are generally poor conductors of electricity.
- They do not contain free-moving electrons or ions.
- Since electrical conductivity requires charged particles that can move, simple molecular compounds cannot carry current in any state (solid, liquid, or gas).
Exceptions:
- Some substances (like acids in water) may ionize and conduct electricity, but that involves chemical change or ionization.
| Property | Explanation |
|---|---|
| Low melting and boiling points | The molecules are held together by weak intermolecular forces. These require little energy to overcome, so the substance melts or boils easily. |
| Poor electrical conductivity | There are no free electrons or ions, so the substance cannot carry an electric current. |
| Soft when solid | The weak forces between molecules mean they can be easily separated, so the solid is soft or easily broken. |
| Solubility | Some molecular substances dissolve in water or organic solvents, depending on the type of molecule and solvent. |
Example Substances:
- CO2: Gas at room temperature, sublimes at -78°C
- H2O: Liquid, boils at 100°C — slightly higher due to hydrogen bonding
- Cl2: Yellow-green gas at room temperature, low boiling point
- CH4: Gas with a very low boiling point (−161°C)
Example
Explain in detail why \( \text{H}_2\text{O} \) (water) shows the typical properties of a simple molecular substance, and mention any exceptions due to hydrogen bonding.
▶️Answer/Explanation
1. Structure and Bonding:
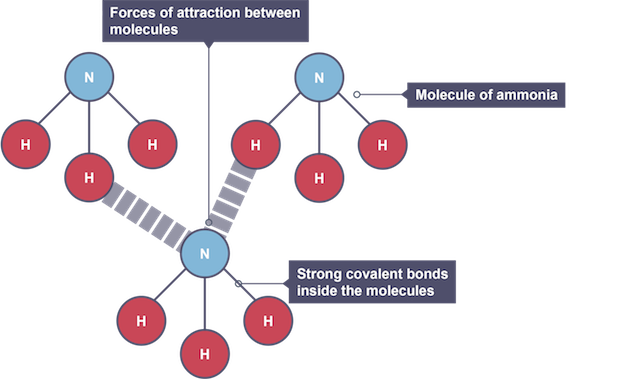
Water molecules consist of two hydrogen atoms covalently bonded to one oxygen atom, forming \( \text{H}_2\text{O} \). Inside each molecule, the covalent bonds are very strong and do not break during melting or boiling. However, the forces between water molecules are weaker compared to covalent bonds. These are mainly hydrogen bonds, which are stronger than normal van der Waals forces but still much weaker than covalent bonds.
2. Melting and Boiling Points:
Like other simple molecular substances, water would be expected to have low melting and boiling points because only the intermolecular forces need to be overcome during a phase change. However, water’s boiling point is unusually high for a small molecule (100°C) because of the presence of hydrogen bonding between molecules. Hydrogen bonds require more energy to break compared to weak van der Waals forces. This is why water is a liquid at room temperature, whereas other similar-sized molecules such as \( \text{CH}_4 \) (methane) are gases.
3. Electrical Conductivity:
Pure water does not have free-moving electrons or ions, so it cannot conduct electricity well. Simple molecular substances like \( \text{H}_2\text{O} \) lack mobile charged particles. However, water can conduct electricity slightly if ions are present (for example, when salts or acids are dissolved), but that involves a chemical change introducing ions.
4. Softness and Physical State:
In its solid state (ice), water is relatively soft compared to ionic or metallic solids. The weak intermolecular hydrogen bonds between molecules allow the molecules to be separated easily, giving ice a low hardness and low density compared to most solids. The open lattice structure due to hydrogen bonding makes ice less dense than liquid water.
5. Solubility:
Water itself is a solvent, but its behavior also reflects typical molecular solubility rules. Polar molecular substances tend to dissolve well in water due to dipole-dipole attractions and hydrogen bonding, whereas non-polar simple molecular substances (like \( \text{Cl}_2 \) or \( \text{CH}_4 \)) are poorly soluble in water because there are no favorable interactions with polar water molecules.
Conclusion:
\( \text{H}_2\text{O} \) is an excellent example of a simple molecular substance. It demonstrates weak intermolecular forces compared to covalent bonds, low electrical conductivity, and relatively low hardness. The main exception is its unusually high boiling and melting points due to hydrogen bonding, which is stronger than typical van der Waals forces found in many other simple molecular substances.
