Water- CIE iGCSE Chemistry Notes - New Syllabus
Water for iGCSE Chemistry Notes
Core Syllabus
- Describe chemical tests for the presence of water using anhydrous cobalt(II) chloride and anhydrous copper(II) sulfate
- Describe how to test for the purity of water using melting point and boiling point
- Explain that distilled water is used in practical chemistry rather than tap water because it contains fewer chemical impurities
- State that water from natural sources may contain substances, including:
(a) dissolved oxygen
(b) metal compounds
(c) plastics
(d) sewage
(e) harmful microbes
(f) nitrates from fertilisers
(g) phosphates from fertilisers and detergents - State that some of these substances are beneficial, including:
(a) dissolved oxygen for aquatic life
(b) some metal compounds provide essential minerals for life - State that some of these substances are potentially harmful, including:
(a) some metal compounds are toxic
(b) some plastics harm aquatic life
(c) sewage contains harmful microbes which cause disease
(d) nitrates and phosphates lead to deoxygenation of water and damage to aquatic life
Details of the eutrophication process are not required - Describe the treatment of the domestic water supply in terms of:
(a) sedimentation and filtration to remove solids
(b) use of carbon to remove tastes and odours
(c) chlorination to kill microbes
Detecting and testing water
Chemical Tests for the Presence of Water
Chemists can detect the presence of water using specific chemical tests that involve colour changes of certain compounds. Two common tests are with anhydrous cobalt(II) chloride and anhydrous copper(II) sulfate.
Test with anhydrous cobalt(II) chloride:
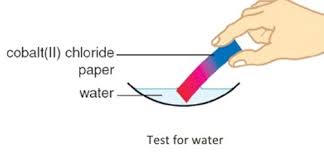
- Anhydrous cobalt(II) chloride is blue in colour.
- When a few drops of water are added, it reacts and changes colour to pink because it forms the hydrated compound \(\text{CoCl}_2·6\text{H}_2\text{O}\).
- This test is very sensitive and can detect small amounts of water.
Test with anhydrous copper(II) sulfate:
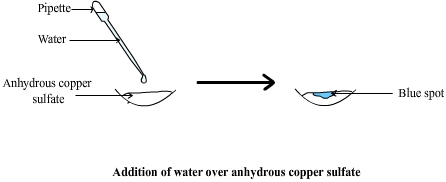
- Anhydrous copper(II) sulfate is white in colour.
- When water is added, it forms the hydrated compound \(\text{CuSO}_4·5\text{H}_2\text{O}\), which is blue.
- This test is widely used in laboratories to quickly detect water.
Example
How can you confirm that a solid sample contains water using cobalt(II) chloride?
▶️Answer/Explanation
- Take a small amount of anhydrous cobalt(II) chloride (blue).
- Add a few drops of the sample or expose it to water vapour.
- If the solid turns pink, it indicates that water is present.
Example
Explain the colour change observed when anhydrous copper(II) sulfate reacts with water.
▶️Answer/Explanation
- Anhydrous CuSO4 is white and reacts with water to form the hydrated compound CuSO4·5H2O.
- The hydrated compound is blue in colour, indicating the presence of water.
- This is a simple and reliable laboratory test for water.
Testing the Purity of Water
The purity of water can be determined by measuring its melting point and boiling point. Pure water has very specific physical properties that change if impurities are present.
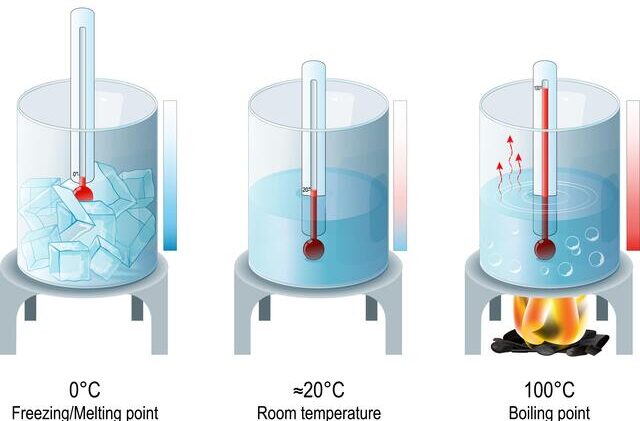
Melting point test:
- Pure water melts at exactly 0°C.
- To test, cool the water sample and record the temperature at which it begins to solidify.
- If impurities are present, the freezing point is lowered (freezing point depression).
- This method is sensitive to dissolved salts and other substances.
Boiling point test:
- Pure water boils at exactly 100°C at standard atmospheric pressure.
- Heat the water sample and note the temperature at which it boils.
- Impurities raise the boiling point (boiling point elevation), so the water boils at a temperature higher than 100°C.
- This test can indicate the presence of dissolved salts or other substances.
Example
A water sample freezes at -1.5°C. What does this indicate about its purity?
▶️Answer/Explanation
- The melting point is lower than 0°C, which indicates the water contains impurities.
- Dissolved salts or other substances are causing freezing point depression.
- The water is not pure.
Example
A water sample boils at 102°C. What does this suggest?
▶️Answer/Explanation
- The boiling point is higher than 100°C, which indicates the presence of dissolved impurities.
- Boiling point elevation is caused by dissolved salts or other substances.
- The water is not pure and may not be suitable for sensitive experiments.
Use of Distilled Water in Practical Chemistry
In practical chemistry, distilled water is preferred over tap water because it contains far fewer chemical impurities that could interfere with experiments or reactions.
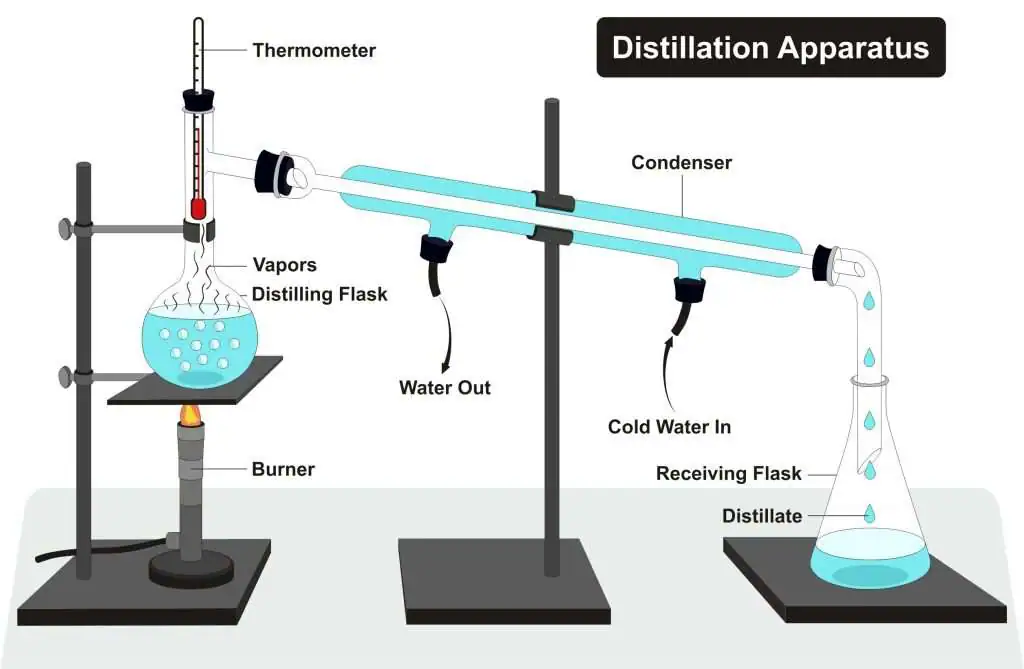
Definition of distilled water:
- Distilled water is water that has been purified by distillation, a process where water is boiled to produce vapour and then condensed back into liquid.
- This process removes dissolved salts, minerals, and most other impurities.
Problems with tap water:
- Tap water contains dissolved minerals such as calcium and magnesium ions.
- It may also contain traces of other chemical substances or ions from plumbing.
- Impurities in tap water can react with chemicals in experiments, leading to inaccurate results or unwanted side reactions.
Advantages of using distilled water:
- Ensures more accurate and reproducible experimental results.
- Prevents interference with chemical reactions caused by ions or impurities.
- Essential for preparing solutions with precisely known concentrations.
Example
Why should distilled water be used instead of tap water to prepare a standard solution of sodium chloride?
▶️Answer/Explanation
- Tap water contains dissolved ions such as calcium and magnesium.
- These impurities could react with sodium chloride or interfere with the measurement of concentration.
- Using distilled water ensures the solution has an accurate, known concentration.
Example
Explain why distilled water is preferred for chemical reactions in the laboratory.
▶️Answer/Explanation
- Distilled water contains very few dissolved salts or impurities.
- This prevents unwanted side reactions and ensures the chemicals react as intended.
- It improves the accuracy and reproducibility of experimental results.
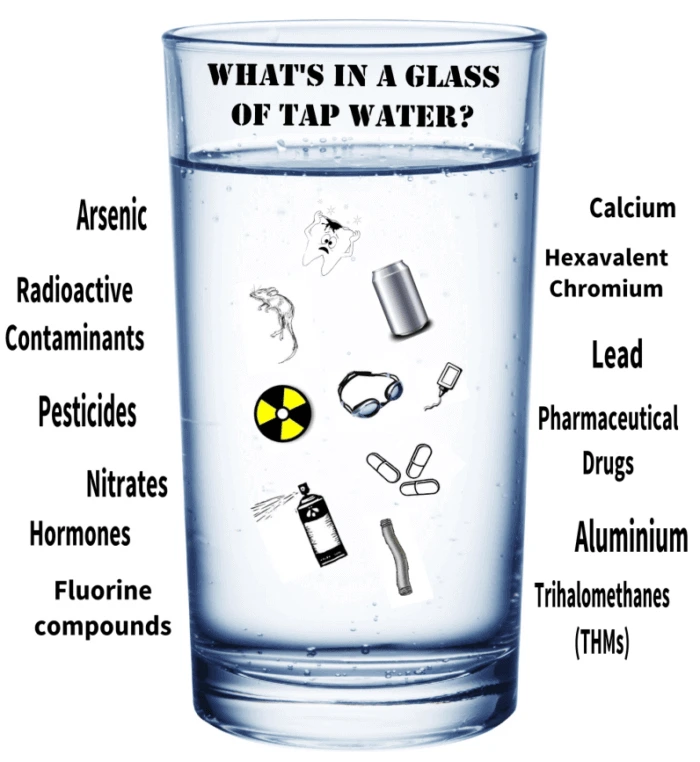
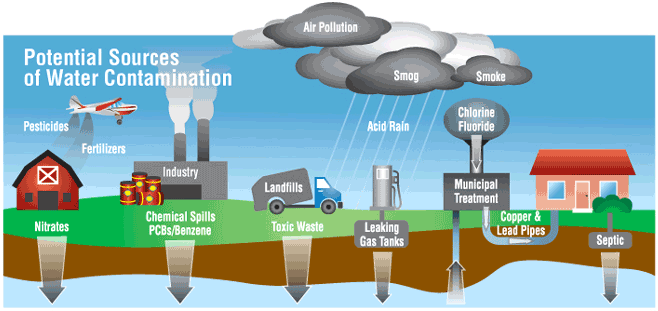
Substances in natural water sources
Substances Present in Natural Water Sources
Water from rivers, lakes, or underground sources may contain a variety of substances. These can come from natural processes, human activities, or runoff from land.
Beneficial Substances in Water
Some substances naturally present in water are essential for life or provide benefits to ecosystems and humans.
Dissolved oxygen (O2):
- Essential for aquatic organisms, including fish, insects, and microorganisms.
- Supports respiration and maintains healthy ecosystems in rivers, lakes, and oceans.
- High oxygen levels indicate good water quality.
Essential metal compounds:
- Water may contain dissolved ions such as calcium (Ca²⁺), magnesium (Mg²⁺), and iron (Fe²⁺/Fe³⁺).
- These metals are essential minerals for human and animal health.
- Calcium and magnesium contribute to the hardness of water, while iron is important for blood formation.
Example
Which substances in natural water are beneficial for aquatic life?
▶️Answer/Explanation
- Dissolved oxygen – necessary for respiration of fish and microorganisms.
- Trace metal ions like calcium and magnesium – contribute to essential minerals in the water.
Example
Explain why calcium and magnesium ions in water can be considered beneficial.
▶️Answer/Explanation
- They provide essential minerals for human and animal health.
- Calcium contributes to strong bones and teeth, magnesium supports enzymes and metabolism.
- These ions also influence water hardness, which can be important for some industrial and biological processes.
Potentially Harmful Substances in Water
Some substances naturally present or introduced into water can be harmful to aquatic life, humans, and the environment.
Toxic metal compounds:
- Certain dissolved metals, such as lead (Pb²⁺), mercury (Hg²⁺), and cadmium (Cd²⁺), are toxic even in small concentrations.
- These can accumulate in aquatic organisms and enter the food chain, causing health problems for humans and animals.
Plastics:
- Microplastics and larger plastic debris can harm aquatic life by ingestion or physical entanglement.
- Plastics are non-biodegradable and persist in the environment for a long time.
Sewage and harmful microbes:
- Sewage introduces bacteria, viruses, and parasites into water bodies.
- These microbes can cause waterborne diseases in humans and animals, such as cholera or dysentery.
Nitrates and phosphates:
- These nutrients come from agricultural runoff and detergents.
- Excess nitrates and phosphates can lead to deoxygenation of water (reduction in dissolved oxygen), harming fish and aquatic organisms.
- This can disrupt ecosystems and reduce biodiversity, although the detailed eutrophication process is not required at this level.
Example
Why are high levels of nitrates in water harmful to aquatic life?
▶️Answer/Explanation
- Excess nitrates promote the overgrowth of algae and plants.
- When these die and decompose, dissolved oxygen levels in water drop (deoxygenation).
- Low oxygen levels can kill fish and other aquatic organisms.
Example
Explain why sewage in water can be dangerous for humans.
▶️Answer/Explanation
- Sewage contains harmful microbes, including bacteria and viruses.
- These microbes can cause waterborne diseases such as cholera, typhoid, and dysentery.
- Untreated sewage in water makes it unsafe for drinking, cooking, and recreational use.
Example
What are the risks of toxic metal ions in natural water?
▶️Answer/Explanation
- Toxic metals like lead, mercury, and cadmium can accumulate in aquatic organisms.
- They enter the food chain and may cause serious health problems in humans and animals, including organ damage and developmental issues.
- Even small concentrations can be hazardous over time.
Treatment of Domestic Water Supply
Treatment of Domestic Water Supply
Water from natural sources often contains impurities that must be removed before it is safe for domestic use. Treatment processes remove solids, tastes, odours, and harmful microbes.
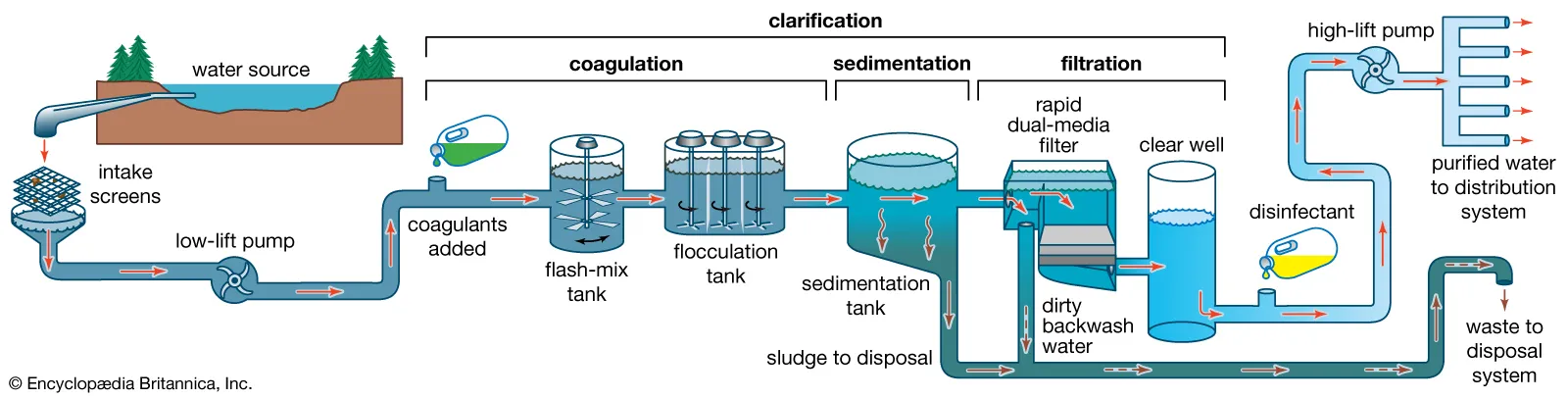
Sedimentation and filtration:
- Sedimentation allows heavier solid particles to settle at the bottom of tanks or reservoirs.
- Filtration passes water through layers of sand, gravel, or other materials to remove smaller suspended particles.
- This process ensures that water is clear and free from visible debris.
Use of carbon to remove tastes and odours:
- Activated carbon or charcoal absorbs dissolved substances that cause unpleasant tastes or odours.
- Common substances removed include chlorine, organic matter, and certain pollutants.
- This step improves the sensory quality of water without altering its chemical composition.
Chlorination to kill microbes:
- Chlorine or chlorine compounds are added to water to kill harmful bacteria, viruses, and other pathogens.
- This ensures the water is safe to drink and reduces the risk of waterborne diseases.
- Chlorination is a widely used disinfection method in domestic water supplies.
Example
Explain how sedimentation and filtration improve water quality.
▶️Answer/Explanation
- Sedimentation allows heavy solids to settle at the bottom of the tank.
- Filtration removes smaller suspended particles as water passes through sand or gravel.
- This produces clear water free from visible debris.
Example
Why is activated carbon used in water treatment?
▶️Answer/Explanation
- Activated carbon absorbs dissolved substances that cause bad taste or odour.
- It removes pollutants such as chlorine and organic compounds, improving water quality.
