CIE iGCSE Co-Ordinated Science C4.1 Electrolysis Exam Style Questions Paper 2
Question
Which statements about the electrolysis of aqueous copper(II) sulfate are correct?
1 Oxygen is produced at the anode when carbon electrodes are used.
2 Copper is produced at the cathode when copper electrodes are used.
3 Hydrogen is produced at the cathode when carbon electrodes are used.
4 Electrolysis does not occur when inert electrodes are used.
(A) 1 and 2
(B) 1 and 4
(C) 2 and 3
(D) 3 and 4y
▶️Answer/Explanation
Ans: A
Question
The diagram shows the electrolysis of molten lead(II) bromide using inert electrodes.
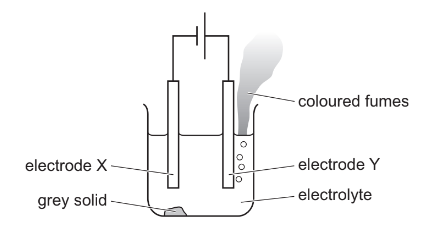
Which statement about this experiment is correct?
(A) Electrode X is positively charged.
(B) The coloured fumes are produced at the negative electrode.
(C) The electrolyte is lead(II) bromide.
(D) The grey solid is lead(II) bromide.
▶️Answer/Explanation
Ans : C
Question
Which statement describes what happens during electrolysis?
(A) Covalent compounds produce more complex substances.
(B) Covalent compounds produce simpler substances.
(C) Ionic compounds produce more complex substances.
(D) Ionic compounds produce simpler substances.
▶️Answer/Explanation
Ans: D
Question
The reactions of four different metals W, X, Y and Z with aqueous solutions of their ions are
listed.
● W reacts with aqueous ions of X and of Z but not with aqueous ions of Y.
● X does not react with aqueous ions of W, Y or Z.
● Y reacts with aqueous ions of W, X and Z.
● Z reacts with aqueous ions of X but not with aqueous ions of Y or W.
Which row shows the tendency of the metals to form positive ions?
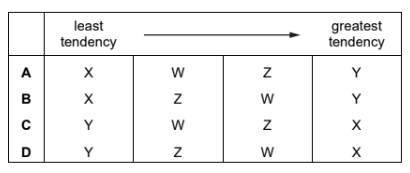
▶️Answer/Explanation
Ans: B
