CIE iGCSE Co-ordinated Sciences-B5 Enzymes - Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-B5. Enzymes – Study Notes
CIE iGCSE Co-ordinated Sciences-B5. Enzymes – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
- Describe enzymes as proteins that are involved in all metabolic reactions, where they function as biological catalysts
- Investigate and describe the effect of changes in temperature and pH on enzyme activity
Supplement
- Describe and explain enzyme action with reference to: the active site, enzyme–substrate complex, substrate and product
- Describe and explain the specificity of enzymes in terms of the complementary shape and fit of the active site with the substrate
- Explain the effect of changes in temperature on enzyme activity in terms of kinetic energy, shape and fit, frequency of effective collisions and denaturation
- Explain the effect of changes in pH on enzyme activity in terms of shape and fit and denaturation
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Enzymes – Biological Catalysts
📌 Definition
Enzymes are proteins that act as biological catalysts.
- They speed up metabolic reactions without being used up.
- Needed only in small amounts.
🔑 Key Features of Enzymes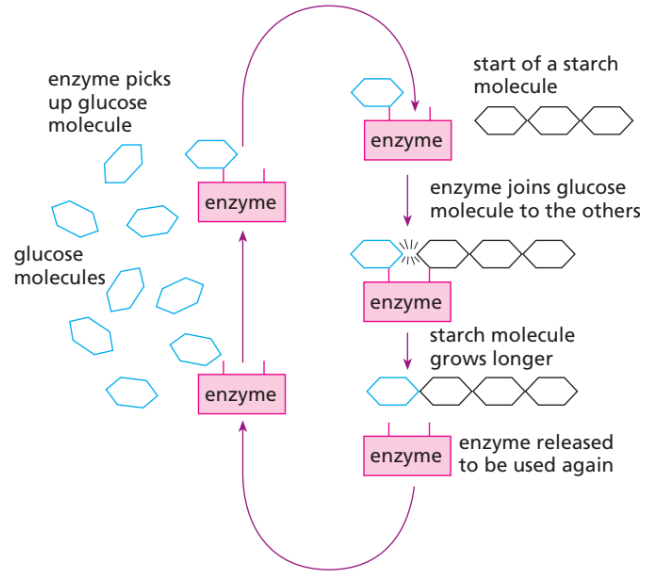
- Made inside all living cells.
- Work on a specific substrate (lock-and-key fit).
- Can be used again and again.
- Make reactions much faster (e.g. catalase breaks down ~40,000 hydrogen peroxide molecules per second!).
🧩 Enzyme Action (Lock & Key Model)
- Substrate has a specific shape.
- Enzyme has an active site that is complementary to that substrate.
- Substrate binds to active site → forms enzyme–substrate complex.
- Reaction occurs → product is formed.
- Product is released → enzyme is free for another reaction.
👉 Works for both:
- Building-up reactions (anabolic) → e.g. glucose + glucose → maltose, or many glucose → starch/cellulose.
- Breaking-down reactions (catabolic) → e.g. starch → maltose, hydrogen peroxide → water + oxygen.
🌡️ Effect of Temperature on Enzyme Activity
- Low temp → enzymes inactive (molecules have little kinetic energy).
- Optimum temp (usually ~37 °C in humans) → fastest activity.
- High temp → enzyme denatures (active site shape changes, substrate no longer fits).
⚖️ Effect of pH on Enzyme Activity
- Each enzyme works best at an optimum pH.
- Example: Pepsin (stomach enzyme) works best at pH 2.
- Too acidic/alkaline → enzyme denatures (active site shape changes).
📊 Summary Table
| Feature | Explanation |
|---|---|
| Nature | Protein |
| Function | Biological catalyst |
| Specificity | Substrate fits into enzyme’s active site (lock & key) |
| Reactions | Can build up (anabolic) or break down (catabolic) molecules |
| Temperature | Optimum → maximum activity; too high → denaturation |
| pH | Optimum required; extreme pH → denaturation |
| Reuse | Not used up, can work repeatedly |
📝 Quick Recap
Enzyme = protein catalyst
Lock & Key fit → substrate + enzyme = product
Temperature & pH affect activity
Denatured enzyme = useless (active site loses shape)
Examples:
Catalase → breaks down hydrogen peroxide
Amylase → starch → maltose
Effect of Temperature & pH on Enzyme Activity
📌 Introduction
Enzymes are proteins, so their activity depends strongly on temperature and pH. Both factors affect the shape of the enzyme’s active site, which is essential for binding substrates.
🌡️ Enzymes & Temperature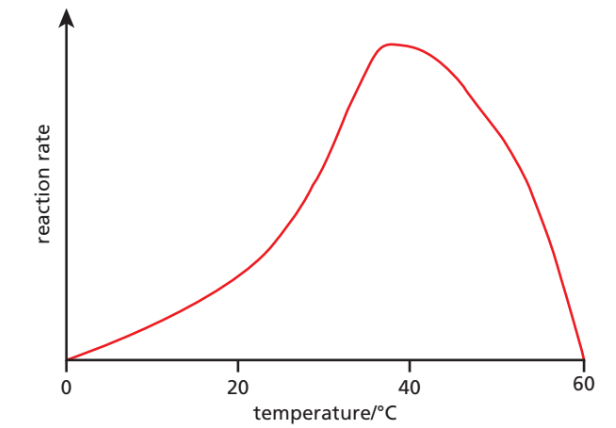
- Increasing temperature → molecules gain kinetic energy → more enzyme–substrate collisions → faster reaction.
- Optimum temperature: ~37 °C in humans (varies in organisms).
- Above optimum:
- Proteins lose shape → active site deforms.
- Substrate no longer fits → reaction rate falls.
- Around 50 °C and above → enzymes become denatured (irreversible loss of shape).
- Example: Egg white (albumin protein) turning solid when cooked = protein denaturation.
⚖️ Enzymes & pH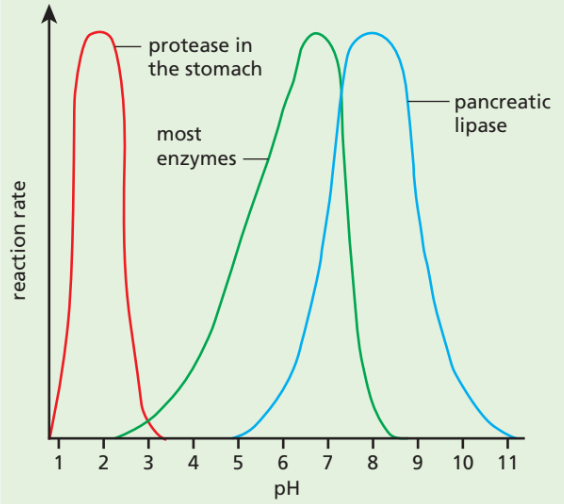
- Each enzyme has an optimum pH (best activity).
- Example:
- Pepsin (stomach protease): works at pH 2 (acidic).
- Amylase (saliva): works best at neutral pH 7.
- Lipase (pancreas): works best in slightly alkaline pH 8.
- Deviations from optimum pH:
- Change in hydrogen ion concentration alters bonds in enzyme structure.
- Active site loses its complementary shape.
- Enzyme activity decreases.
- Extreme pH → irreversible denaturation.
- Mild changes in pH → effect usually reversible when pH returns to normal.
📊 Summary Table
| Factor | Effect on Enzyme Activity | Example |
|---|---|---|
| Temperature ↑ (till optimum) | Faster reactions (more collisions) | Human enzymes: ~37 °C optimum |
| Too high temperature (>50 °C) | Denaturation → enzyme stops working | Cooking egg white |
| Low/high pH (away from optimum) | Slower reactions (distorted active site) | Amylase inactive at pH 2 |
| Optimum pH | Maximum enzyme activity | Pepsin → pH 2, Lipase → pH 8 |
📝 Quick Recap
Temp ↑ → faster → optimum → denatured.
pH too high/low → disrupts enzyme structure.
Optimum temp ≈ 37 °C, optimum pH depends on enzyme.
Denaturation = permanent change in shape → enzyme useless.
Enzyme Action
📌 Introduction
Enzymes are biological catalysts (proteins) that speed up reactions without being used up. They work on specific substances called substrates and convert them into products.
🔑 Key Concepts
1. Active Site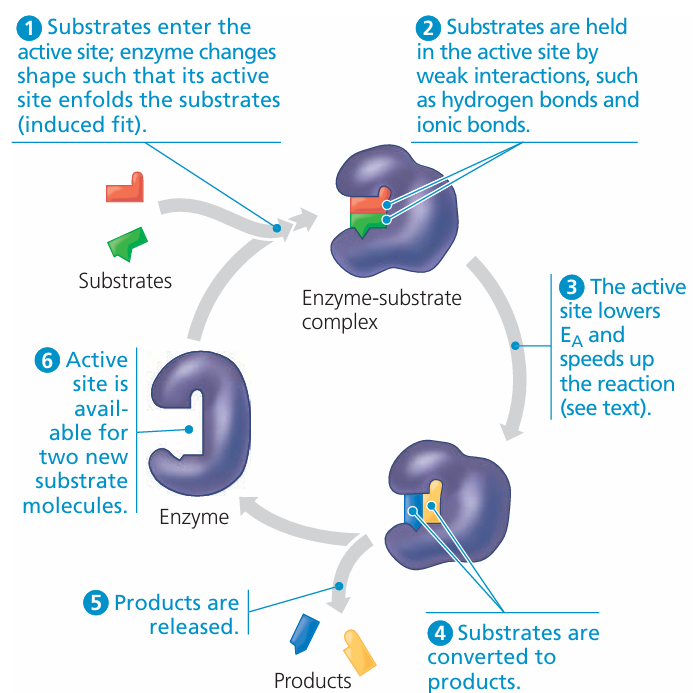
- Small region on enzyme where the substrate binds.
- Has a specific shape complementary to the substrate.
- Responsible for enzyme specificity (lock-and-key idea).
2. Substrate
- The reactant molecule(s) that the enzyme acts upon.
- Fits into the active site like a key fits a lock.
3. Enzyme-Substrate Complex
- Temporary structure formed when substrate binds to active site.
- Helps weaken bonds in substrate → lowers activation energy → reaction proceeds faster.
4. Product
- After reaction, the substrate is converted into product(s).
- Product no longer fits the active site → released → enzyme free to act again.
⚙️ Stepwise Mechanism
- Substrate collides with enzyme.
- Substrate binds to enzyme’s active site → forms enzyme–substrate complex.
- Reaction occurs → substrate converted into product.
- Product is released.
- Enzyme remains unchanged → can be reused.
📊 Summary Table
| Term | Explanation | Example |
|---|---|---|
| Active site | Special region on enzyme binding substrate | Catalase active site binds H₂O₂ |
| Substrate | Molecule acted upon by enzyme | Hydrogen peroxide (H₂O₂) |
| Enzyme–substrate complex | Temporary binding during reaction | Catalase + H₂O₂ |
| Product | End result of reaction | Water + Oxygen from H₂O₂ breakdown |
📝 Quick Recap
Active site = specific shape for substrate.
Substrate binds → enzyme–substrate complex forms.
Reaction occurs → products released.
Enzyme remains unchanged, ready for reuse.
Specificity of Enzymes
📌 Introduction
Enzymes are highly specific – each enzyme only works with one type (or a few types) of substrate. This specificity comes from the unique shape of the active site.
🔑 Explanation of Specificity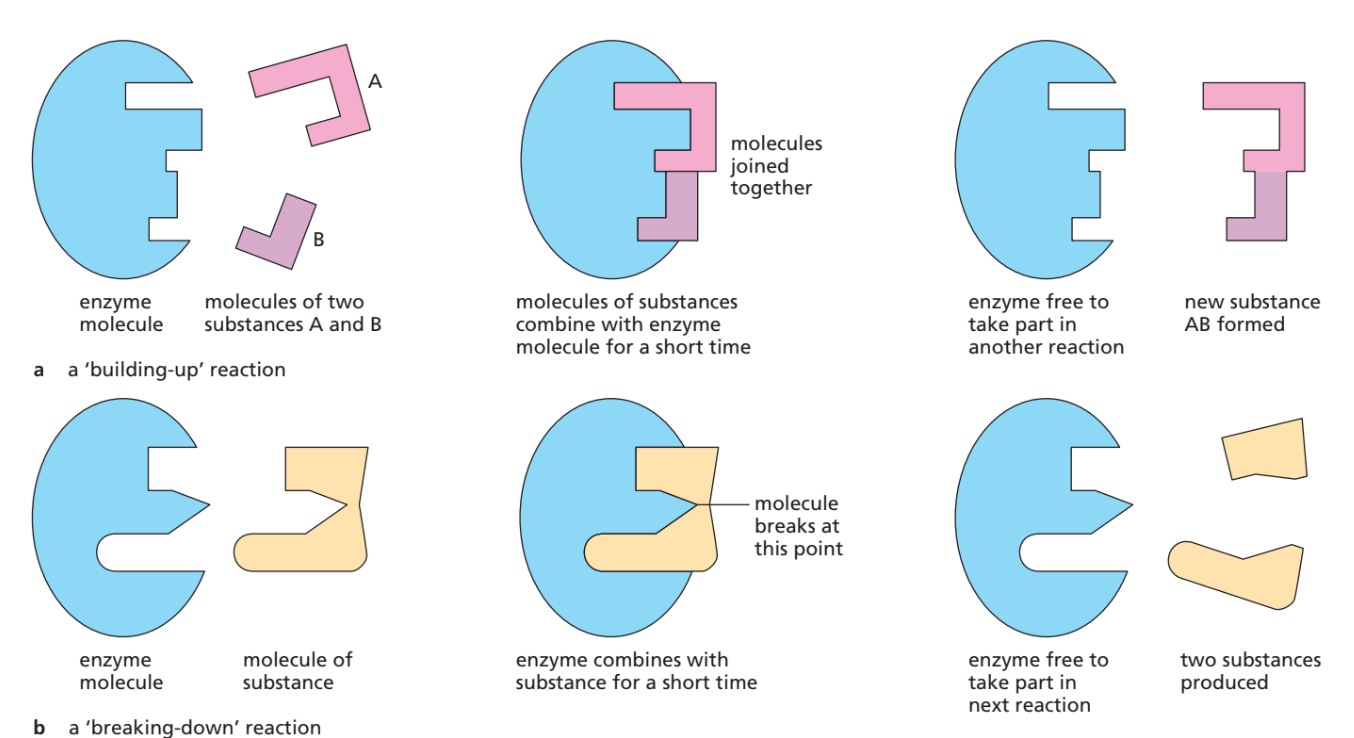
1. Active Site Shape
- Enzymes are proteins → folded into precise 3D shapes.
- The active site has a unique structure that only fits certain substrate molecules.
2. Complementary Fit
- Substrate fits into active site like a key fits a lock (Lock-and-Key model).
- Shapes are complementary, allowing binding and reaction.
3. Enzyme–Substrate Complex Formation
- Only correctly shaped substrates can bind.
- Binding lowers activation energy → reaction occurs.
4. Products Formation
- Substrate converted into product(s).
- Active site releases product → enzyme is free to be reused.
📊 Summary Table
| Concept | Explanation |
|---|---|
| Specificity | Enzyme only catalyses one type of reaction. |
| Reason | Active site has a unique shape matching only one substrate. |
| Lock-and-Key Model | Substrate = key, Active site = lock. |
| Result | Ensures reactions are controlled and efficient. |
📝 Quick Recap
Enzyme specificity = only the right substrate fits.
Active site shape is complementary to substrate.
Wrong-shaped molecules cannot bind → no reaction.
Lock-and-key analogy explains this fit.
Effect of Temperature on Enzyme Activity
📌 Introduction
Enzymes are proteins, and their activity depends on temperature. Changes in temperature affect kinetic energy, shape of active site, and collision frequency between enzyme and substrate.
🔑 Explanation
1. Low Temperatures
- Enzyme + substrate molecules have low kinetic energy.
- Fewer collisions → fewer enzyme–substrate complexes.
- Reaction rate is slow (but enzymes are not denatured).
2. Increasing Temperature (up to optimum ~37°C in humans)
- Molecules gain more kinetic energy.
- Substrates collide with active sites more frequently and with more energy.
- More effective collisions → more enzyme–substrate complexes.
- Reaction rate increases.
3. Optimum Temperature
- Rate of reaction is at its maximum.
- Enzyme structure is still intact, and collisions are most efficient.
4. Above Optimum
- Enzyme (a protein) starts to lose its shape.
- Hydrogen bonds in enzyme structure break.
- Active site shape changes → substrate no longer fits properly.
- Fewer enzyme–substrate complexes → rate decreases.
5. High Temperatures (>50–60°C)
- Enzyme becomes denatured (permanent change in shape).
- Active site is destroyed → substrate cannot bind.
- Reaction stops completely.
📊 Summary Table
| Temperature | Effect on Enzyme | Reason |
|---|---|---|
| Low (0–10°C) | Very slow reaction | Low kinetic energy, few collisions |
| Moderate (20–37°C) | Increasing rate | More collisions, more enzyme–substrate complexes |
| Optimum (~37°C) | Maximum rate | Best fit + highest collision frequency |
| High (>40°C) | Rate decreases | Enzyme loses shape, active site deforms |
| Very High (>50–60°C) | Reaction stops | Enzyme denatured permanently |
📝 Quick Recap
Low temp → slow (low kinetic energy).
Rising temp → faster (more collisions).
Optimum → max activity.
Above optimum → enzyme loses shape, fewer fits.
Very high temp → denaturation (permanent loss).
Effect of pH on Enzyme Activity
📌 Introduction
Enzymes are proteins with an active site whose shape must match the substrate.
pH affects the charges on amino acids → changes shape + fit of the active site.
Each enzyme has an optimum pH where it works best.
🔑 Explanation
1. Optimum pH
- At this pH, the enzyme’s active site has the perfect shape for the substrate.
- Maximum number of enzyme–substrate complexes form → highest reaction rate.
2. Slightly away from optimum
- Active site shape is altered slightly.
- Substrate fits less well → fewer enzyme–substrate complexes → reaction slows down.
3. Extreme pH (very acidic or very alkaline)
- Ionic and hydrogen bonds in enzyme protein structure are disrupted.
- Active site is deformed → no longer complementary to substrate.
- Enzyme is denatured (permanent loss of function).
📊 Examples
- Pepsin (stomach protease): Optimum pH ~2 (acidic).
- Amylase (saliva): Optimum pH ~7 (neutral).
- Lipase (pancreas): Optimum pH ~8 (slightly alkaline).
📋 Summary Table
| pH Condition | Effect on Enzyme | Why |
|---|---|---|
| Optimum pH | Maximum activity | Active site shape is complementary |
| Slightly off optimum | Activity decreases | Shape altered, fewer fits |
| Extreme acidic/alkaline | Denatured | Bonds disrupted, active site destroyed |
📝 Quick Recap
Enzymes work best at their optimum pH.
pH changes → alter active site shape → less fit with substrate.
Extreme pH → enzyme denatures (permanent loss).
