CIE iGCSE Co-ordinated Sciences- C1.1 Solids, liquids and gases - Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-C1.1 Solids, liquids and gases – Study Notes
CIE iGCSE Co-ordinated Sciences-C1.1 Solids, liquids and gases – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
- State the distinguishing properties of solids, liquids and gases.
- Describe the structure of solids, liquids and gases in terms of particle separation, arrangement and motion.
- Describe changes of state in terms of melting, boiling, evaporating, freezing and condensing.
- Describe the effects of temperature and pressure on the volume of a gas.
Supplement
- Explain changes of state in terms of kinetic particle theory, including the interpretation of heating and cooling curves.
- Explain, in terms of kinetic particle theory, the effects of temperature and pressure on the volume of a gas.
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Properties of Solids, Liquids, and Gases
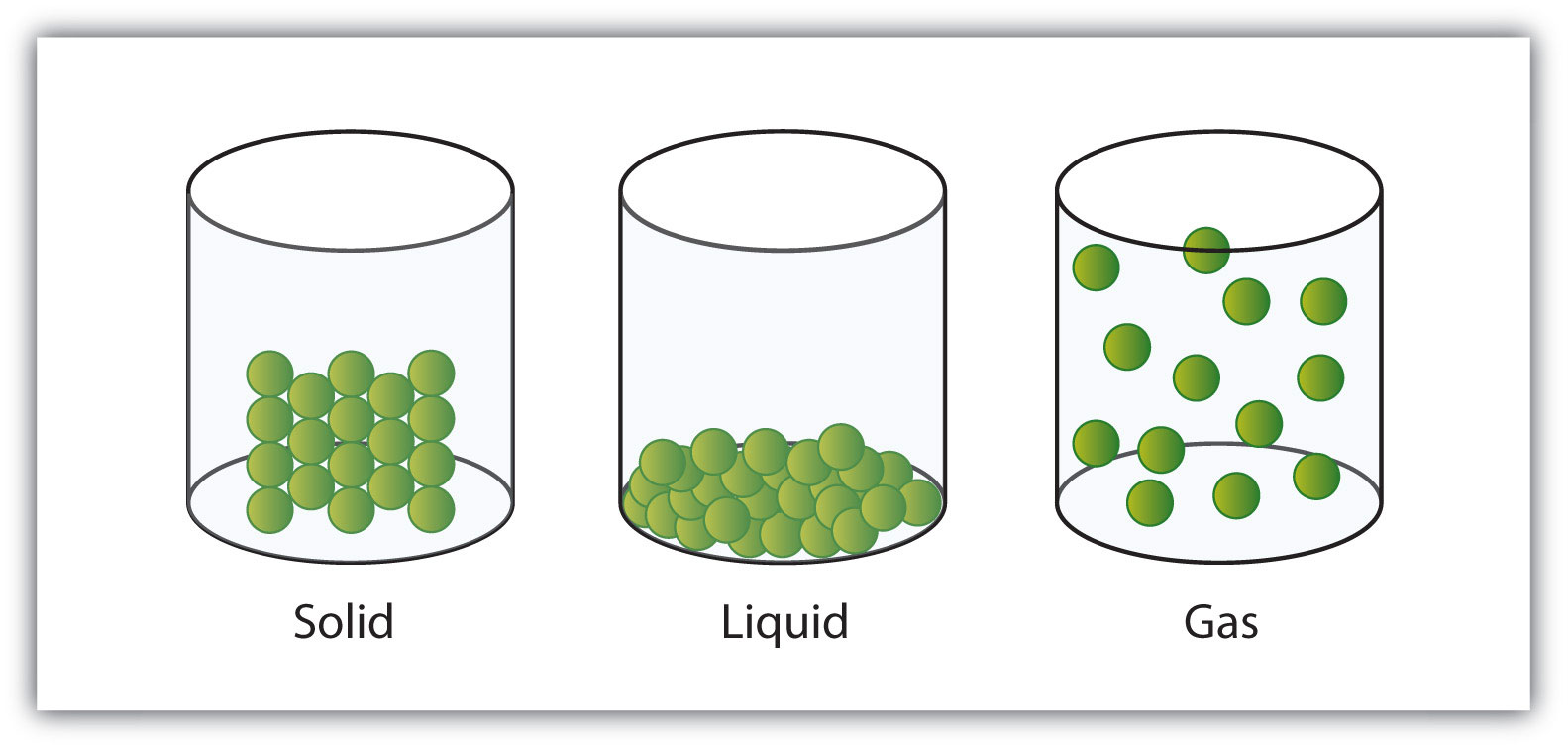
| State of Matter | Distinguishing Properties |
|---|---|
| Solid | • Have a fixed shape • Have a fixed volume • Cannot be compressed • Do not flow |
| Liquid | • No fixed shape (takes shape of container) • Has a fixed volume • Almost incompressible • Flows easily |
| Gas | • No fixed shape (fills container) • No fixed volume (expands to fill container) • Easily compressed • Flows very easily |
Example
Explain why liquids can flow but solids cannot.
▶️Answer/Explanation
In liquids, the particles are close together but not in a fixed arrangement. They can move and slide past one another, allowing liquids to flow.
In solids, the particles are fixed in a regular lattice and can only vibrate in place. They cannot move freely, so solids cannot flow.
Example
Why do gases fill the entire volume of a container while liquids only take the shape of the bottom part of the container?
▶️Answer/Explanation
Gas particles are very far apart and move rapidly in all directions. They spread out to occupy the whole container, giving gases no fixed volume or shape.
Liquids have particles that are close together with limited movement. They cannot expand to fill the whole container, so they only take the shape of the bottom part while keeping a fixed volume.
Structure of Solids, Liquids and Gases
The structure of matter in different states can be described based on three key features: particle separation, arrangement, and motion.
1. Solids
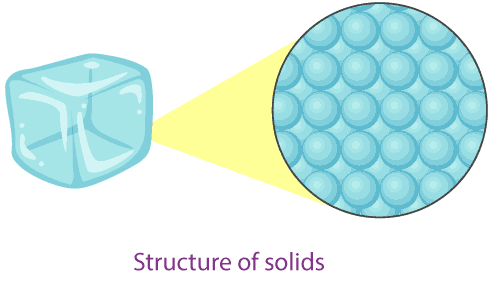
- Particle separation: Particles are very close together with almost no spaces between them.
- Particle arrangement: Particles are arranged in a regular, fixed pattern forming a rigid structure.
- Particle motion: Particles vibrate about fixed positions but cannot move freely.
2. Liquids
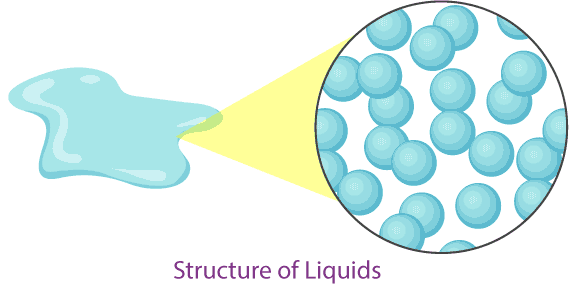
- Particle separation: Particles are close together but with some space to move past one another.
- Particle arrangement: Particles are arranged randomly, not in a fixed pattern.
- Particle motion: Particles can slide and move around each other, allowing liquids to flow and take the shape of their container.
3. Gases
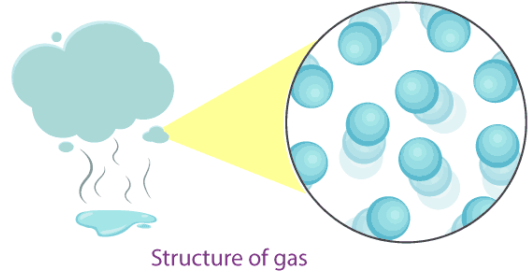
- Particle separation: Particles are far apart with large spaces between them.
- Particle arrangement: Particles are arranged randomly with no fixed pattern.
- Particle motion: Particles move freely and rapidly in all directions, filling the container completely.
Example
Describe the structure of water in its solid, liquid, and gaseous states in terms of particle separation, arrangement, and motion.
▶️Answer/Explanation
Solid (Ice): Particles are closely packed in a regular pattern, vibrating about fixed positions. The ice maintains a definite shape and volume.
Liquid (Water): Particles are close together but arranged randomly. They can move past each other, allowing water to flow and take the shape of its container while maintaining a definite volume.
Gas (Water Vapor/Steam): Particles are far apart and arranged randomly. They move freely and rapidly in all directions, filling the entire container with no definite shape or volume.
Changes of State
Matter can exist in three states: solid, liquid, and gas. A change of state happens when a substance changes from one state to another by gaining or losing energy. These changes are physical changes (no new substance is formed) and are reversible.
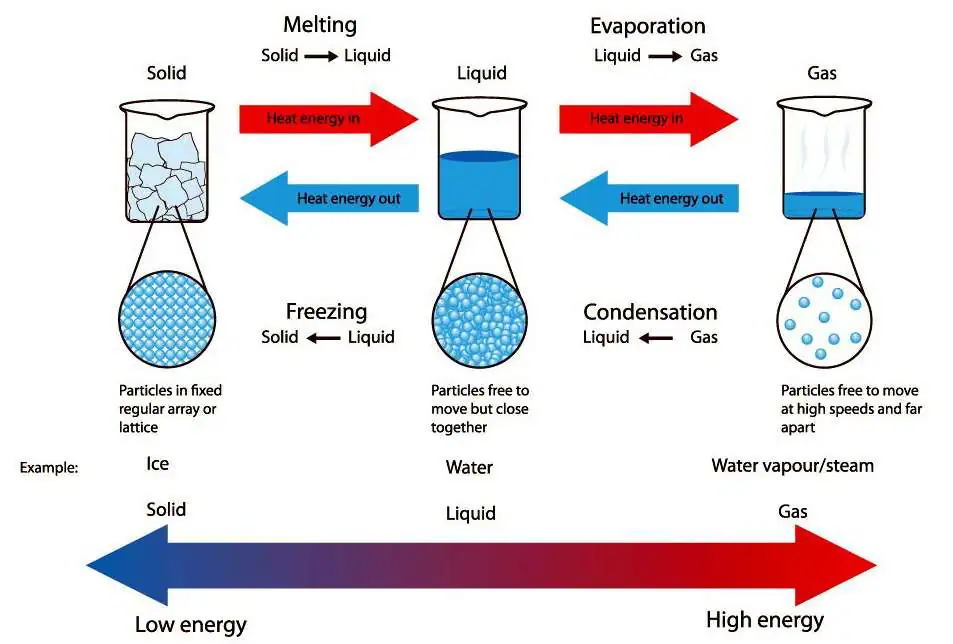
Melting (Solid → Liquid)
- When a solid is heated, its particles gain kinetic energy and vibrate faster.
- At the melting point, the vibrations become strong enough to overcome the strong forces of attraction holding the particles in the fixed lattice.
- The particles break free from their fixed positions and can now slide past one another, forming a liquid.
- The temperature at which this happens is the melting point.
Freezing (Liquid → Solid)
- When a liquid is cooled, the particles lose kinetic energy and move more slowly.
- The attractive forces between particles become stronger compared to their movement.
- Eventually, the particles settle into fixed positions in a regular lattice.
- This forms a solid at the freezing point, which is the same temperature as the melting point for a pure substance.
Boiling (Liquid → Gas)
- When a liquid is heated strongly, the particles gain enough energy to completely overcome the forces of attraction between them.
- At the boiling point, particles throughout the liquid can escape into the gas state, not just from the surface.
- This process happens at a fixed temperature for a pure liquid and is called the boiling point.
Evaporation (Liquid → Gas at surface)
- Unlike boiling, evaporation happens at all temperatures, not just the boiling point.
- Only particles at the surface of the liquid with enough energy to overcome the forces of attraction can escape into the gas state.
- Factors affecting evaporation include temperature, surface area, and air movement.
Condensation (Gas → Liquid)
- When a gas is cooled, its particles lose kinetic energy and slow down.
- The forces of attraction between particles become strong enough to pull them closer together.
- The particles cluster and form a liquid.
Key Points:
- All changes of state are physical changes and reversible.
- Energy is absorbed when particles move apart (melting, boiling, evaporation).
- Energy is released when particles come closer (freezing, condensation).
Example
Describe what happens to the particles of ice as it melts into liquid water.
▶️Answer/Explanation
When ice is heated, the particles (molecules of water) gain kinetic energy and vibrate faster about their fixed positions. At 0°C (the melting point of water), the vibrations become strong enough to overcome the forces of attraction holding the particles in the fixed lattice structure of ice.
The regular lattice breaks down, and the particles are now free to move and slide past one another while still remaining close together. This forms liquid water.
Effects of Temperature and Pressure on the Volume of a Gas
The volume of a gas depends on both its temperature and pressure. These relationships can be explained using the particle model of gases.
1. Effect of Temperature on Gas Volume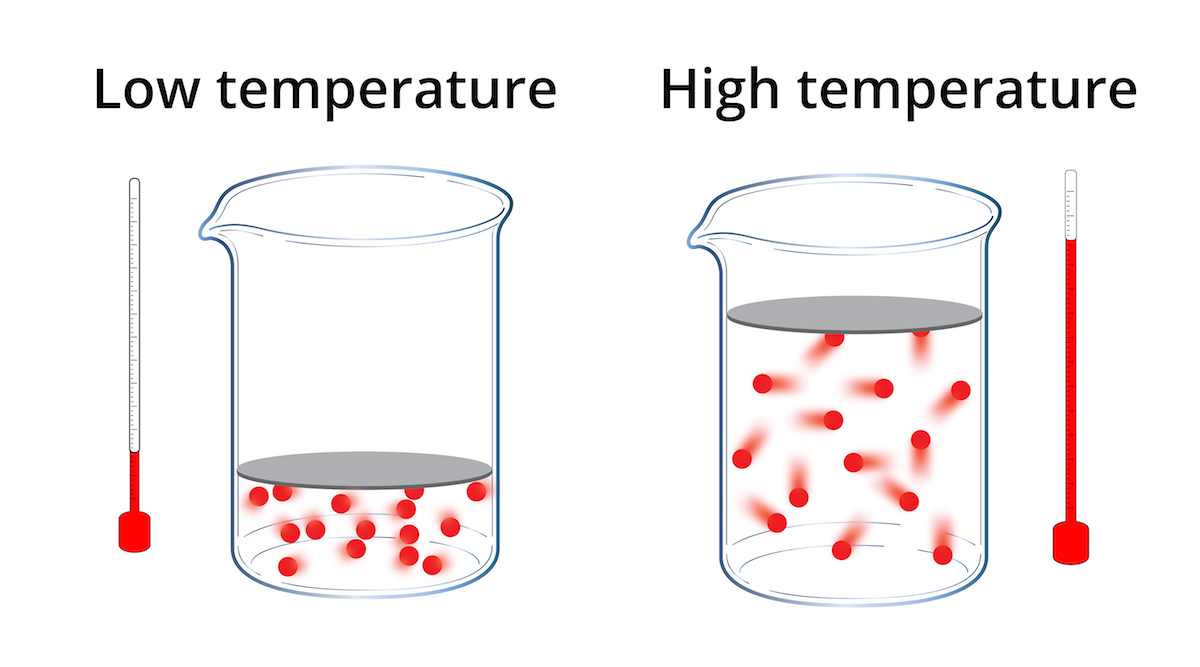
- When a gas is heated, its particles gain kinetic energy and move faster.
- The particles collide with the walls of the container more frequently and with greater force.
- If the pressure is kept constant, the gas will expand its volume increases.
- If the gas is cooled, particles lose energy and move more slowly, so the volume decreases.
Mathematical Relationship (at constant pressure):
\(\mathrm{\dfrac{V_1}{T_1} = \dfrac{V_2}{T_2}}\)
(Temperature must be in kelvins, K)
Conclusion: Gas volume is directly proportional to temperature (in kelvins).
2. Effect of Pressure on Gas Volume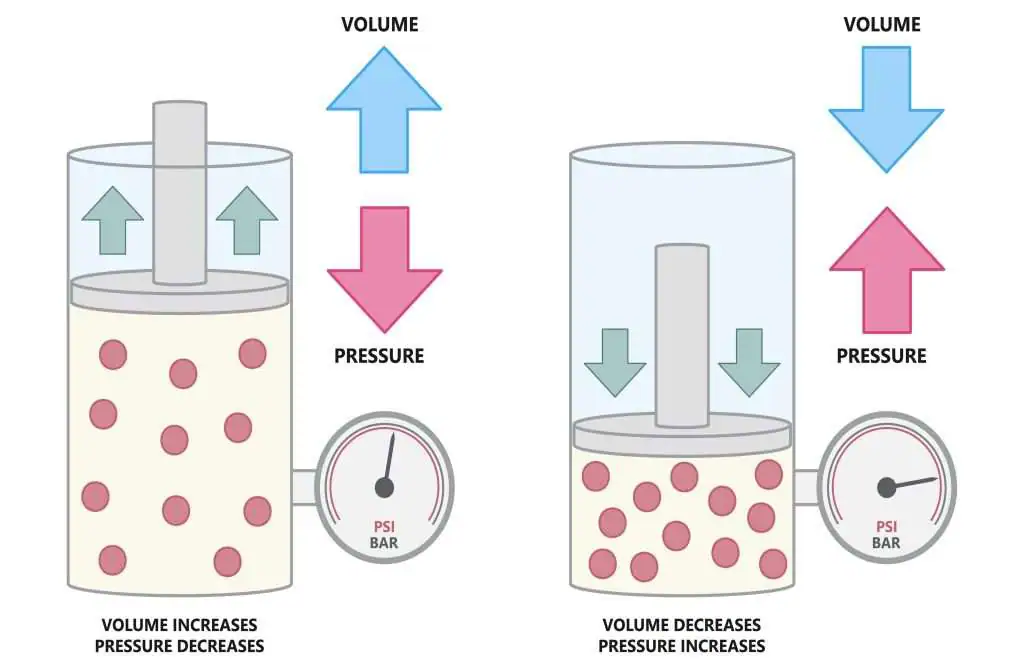
- When the pressure increases, gas particles are forced closer together, so the volume decreases.
- When the pressure decreases, particles move further apart and the volume increases.
- This happens because gas particles are compressible and there is a lot of space between them.
Mathematical Relationship (at constant temperature):
\(\mathrm{P_1V_1 = P_2V_2}\)
Conclusion: Gas volume is inversely proportional to pressure (if temperature is constant).
Summary:
- ↑ Temperature → ↑ Volume (direct relationship)
- ↑ Pressure → ↓ Volume (inverse relationship)
Example :
A gas has a volume of 200 cm³ at 300 K. What will be its volume at 600 K if the pressure remains constant?
▶️ Answer/Explanation
Step 1: Use the formula \(\mathrm{\dfrac{V_1}{T_1} = \dfrac{V_2}{T_2}}\).
Step 2: Substitute: \(\mathrm{\dfrac{200}{300} = \dfrac{V_2}{600}}\).
Step 3: Rearrange: \(\mathrm{V_2 = \dfrac{200 \times 600}{300} = 400 \, cm^3}\).
Final Answer: The volume doubles to 400 cm³ when temperature doubles (pressure constant).
Heating and Cooling Curves
Heating Curve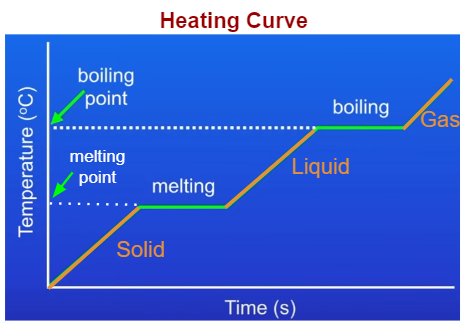
- A heating curve shows how the temperature of a substance changes as it is heated over time.
- When the substance is solid, the temperature rises as particles gain kinetic energy and vibrate faster.
- At the melting point, the temperature stays constant even though heating continues. The energy supplied is used to overcome the strong forces of attraction between particles so that the solid melts into a liquid.
- After all the solid has melted, the temperature of the liquid rises as particles gain more kinetic energy.
- At the boiling point, the temperature again remains constant. The energy supplied is used to completely overcome the forces of attraction so that the liquid changes into gas.
- Once all the liquid has boiled, the temperature of the gas rises as particles move faster with more kinetic energy.
Cooling Curve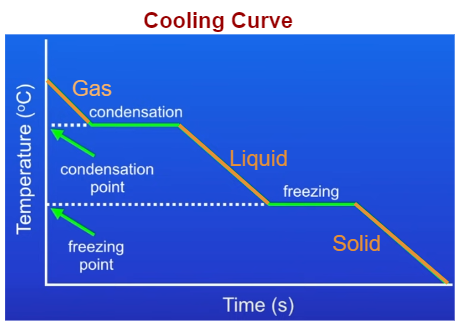
- A cooling curve shows how the temperature of a substance changes as it cools over time.
- When the substance is in the gas state, its temperature falls as particles lose kinetic energy and move more slowly.
- At the condensation point, the temperature stays constant as gas particles lose energy and are pulled closer together to form a liquid.
- After condensation, the liquid temperature continues to fall as particles lose more kinetic energy.
- At the freezing point, the temperature again remains constant while particles lose enough energy for strong forces of attraction to fix them into a regular lattice structure, forming a solid.
- After all the liquid has solidified, the temperature of the solid continues to fall as the particles lose further kinetic energy.
Key Points for Both Curves
- Sloping sections of the curve show temperature change due to change in kinetic energy of particles.
- Flat (horizontal) sections show a change of state. Temperature stays constant because energy is being transferred to break or form intermolecular forces rather than change kinetic energy.
Example
A pure substance is cooled from a hot gas until it becomes a solid. The temperature is recorded and a cooling curve is obtained. Explain why the temperature remains constant at certain points on the curve.
▶️Answer/Explanation
During cooling, the temperature falls as particles lose kinetic energy. However, at the condensation point, the temperature stays constant even though energy is still being removed. This is because energy is released as particles come closer together and form forces of attraction to change the gas into a liquid.
Similarly, at the freezing point, the temperature again remains constant. The energy released is used to form strong forces of attraction between particles, arranging them into a regular lattice to form a solid. Only after the whole substance has solidified does the temperature continue to decrease.
Effects of Temperature and Pressure on the Volume of a Gas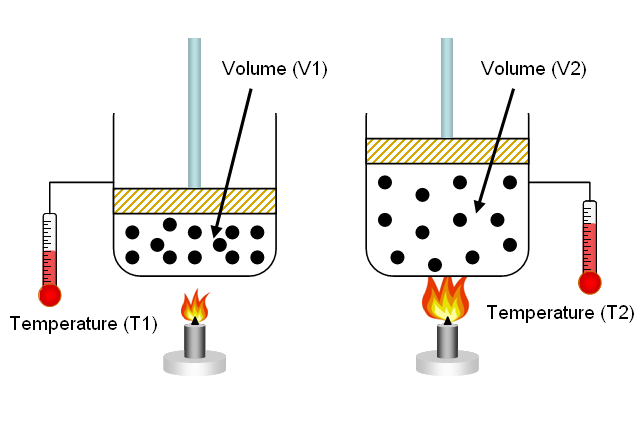
1. Effect of Temperature
- When the temperature of a gas increases, the particles gain kinetic energy and move faster.
- This causes the particles to hit the walls of the container more frequently and with greater force.
- If the pressure is kept constant, the gas expands and its volume increases because the particles push the container walls outward.
- If the temperature decreases, particles move more slowly, collide less forcefully, and the volume decreases (at constant pressure).
2. Effect of Pressure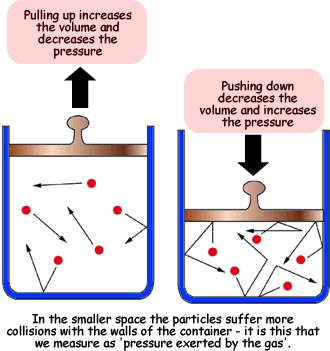
- When the pressure on a gas increases (by reducing container volume), the particles are forced closer together.
- This reduces the volume of the gas since particles have less space to move.
- If pressure decreases (more space is given), the particles spread out and the gas volume increases.
3. Explanation using Kinetic Particle Theory
- Gas particles are far apart and move rapidly in random directions.
- They collide with each other and with the walls of the container, exerting pressure.
- Temperature: Higher temperature → more kinetic energy → faster movement → greater force of collisions → expansion of volume at constant pressure.
- Pressure: Increasing external pressure pushes the particles closer together → volume decreases because particles are compressed into a smaller space.
Key Gas Laws (CGCSE level)
- Charles’s Law: At constant pressure, volume of a gas is directly proportional to its temperature (in Kelvin).
- Boyle’s Law: At constant temperature, volume of a gas is inversely proportional to pressure.
Example
A balloon filled with air is heated while the pressure remains constant. Explain what happens to the volume of the balloon.
▶️Answer/Explanation
When the temperature increases, gas particles gain kinetic energy and move faster. They collide with the balloon walls more forcefully and more frequently. Since the external pressure remains constant, the balloon expands to increase its volume. This agrees with Charles’s Law.
Example
A fixed amount of gas has a volume of 200 cm³ at a pressure of 100 kPa. If the pressure is increased to 250 kPa while the temperature remains constant, calculate the new volume of the gas.
▶️Answer/Explanation
According to Boyle’s Law:
\( P_1 V_1 = P_2 V_2 \)
Substituting the values:
\( 100 \times 200 = 250 \times V_2 \)
\( 20000 = 250 V_2 \)
\( V_2 = \frac{20000}{250} \)
\( V_2 = 80 \, \text{cm}^3 \)
The new volume of the gas is 80 cm³.
Example
A sample of gas occupies 300 cm³ at 27°C (300 K). If the temperature increases to 600 K while pressure remains constant, calculate the new volume of the gas.
▶️Answer/Explanation
According to Charles’s Law:
\( \frac{V_1}{T_1} = \frac{V_2}{T_2} \)
Substituting the values:
\( \frac{300}{300} = \frac{V_2}{600} \)
\( 1 = \frac{V_2}{600} \)
\( V_2 = 600 \, \text{cm}^3 \)
The new volume of the gas is 600 cm³.
