CIE iGCSE Co-ordinated Sciences-C11.1 Formulas and terminology- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-C11.1 Formulas and terminology – Study Notes
CIE iGCSE Co-ordinated Sciences-C11.1 Formulas and terminology – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
- Draw and interpret the displayed formula of a molecule to show all the atoms and all the bonds
- State that a saturated compound has molecules in which all carbon–carbon bonds are single bonds
- State that an unsaturated compound has molecules in which one or more carbon–carbon bonds are not single bonds
Supplement
- State that a homologous series is a family of similar compounds with similar chemical properties
- Describe the general characteristics of a homologous series as:
(a) having the same general formula (recall of specific general formulas is not required)
(b) displaying a trend in physical properties
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Displayed Formulae of Molecules
The displayed formula of a molecule shows all the atoms in the molecule and all the bonds between them, using lines to represent covalent bonds.
Key Features:
- Each line (—) represents a shared pair of electrons (a single covalent bond).
- Atoms are shown by their chemical symbols (e.g. H, C, O).
- Double bonds are shown by two lines (=) and triple bonds by three lines (≡).
Examples:
1. Methane (\(\mathrm{CH_4}\))
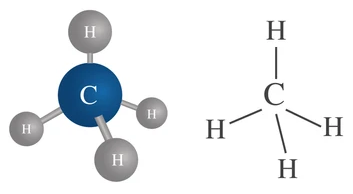
- One carbon atom forms four single covalent bonds with four hydrogen atoms.
- Each bond represents a shared pair of electrons.
2. Water (\(\mathrm{H_2O}\))
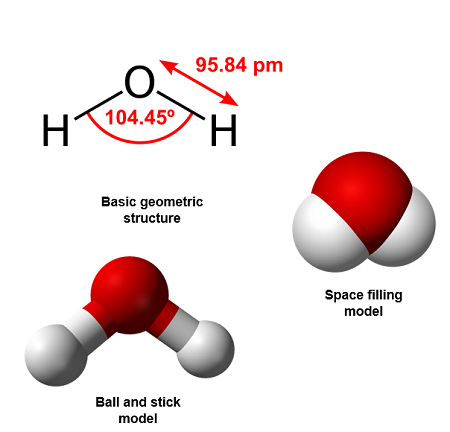
- Oxygen forms two single covalent bonds with two hydrogen atoms.
- Oxygen also has two lone pairs of electrons not shown in the displayed formula.
3. Oxygen (\(\mathrm{O_2}\))
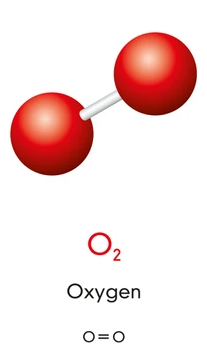
- Each oxygen atom shares two pairs of electrons forming a double bond.
4. Carbon Dioxide (\(\mathrm{CO_2}\))
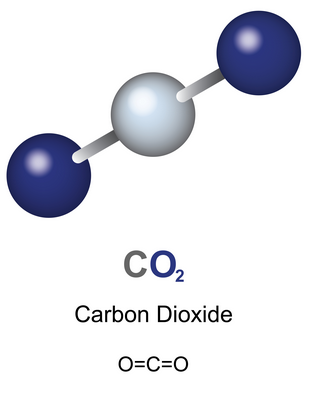
- Carbon forms two double bonds — one with each oxygen atom.
- The displayed formula shows all atoms and all bonds clearly.
Interpreting Displayed Formulae:
- You can determine the number of atoms of each element directly from the diagram.
- You can count the number and type of bonds (single, double, triple) between atoms.
- The displayed formula helps to visualise how atoms are connected within the molecule.
Example :
Draw and describe the displayed formula of ethene (\(\mathrm{C_2H_4}\)).
▶️ Answer/Explanation
Step 1: Ethene has two carbon atoms and four hydrogen atoms.
Step 2: Each carbon shares a double bond with the other carbon and forms two single bonds with hydrogen atoms.
Step 3: Each carbon atom makes four bonds — this satisfies the bonding rules.
Final Answer: The displayed formula of ethene shows all atoms and all covalent bonds, including the double bond between the two carbon atoms.
Saturated Compounds
A saturated compound has molecules in which all carbon–carbon (C–C) bonds are single bonds. 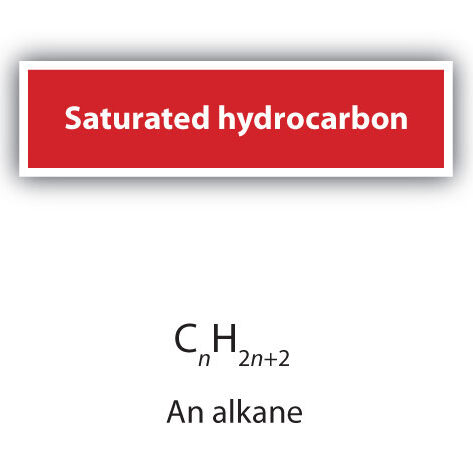
Explanation:
- Saturated compounds contain only single covalent bonds between carbon atoms.
- They cannot add more atoms across a C–C bond because there are no double or triple bonds.
- They belong to the alkane series of hydrocarbons (e.g. methane, ethane, propane).
Example:
Ethane (\(\mathrm{C_2H_6}\)) – a simple saturated hydrocarbon
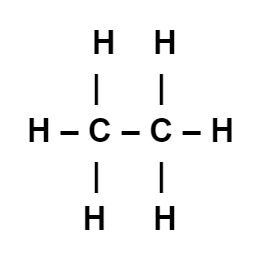
- Each carbon atom forms four single bonds (one with another carbon and three with hydrogen).
- There are no double bonds — so ethane is saturated.
Key Idea: Saturated compounds contain only single C–C bonds and are generally less reactive than unsaturated compounds.
Example :
Explain why ethane is described as a saturated compound.
▶️ Answer/Explanation
Step 1: Ethane (\(\mathrm{C_2H_6}\)) contains only single bonds between its carbon atoms.
Step 2: No double or triple bonds are present.
Final Answer: Ethane is saturated because all its carbon–carbon bonds are single.
Unsaturated Compounds
An unsaturated compound has molecules in which one or more carbon–carbon (C–C) bonds are not single bonds.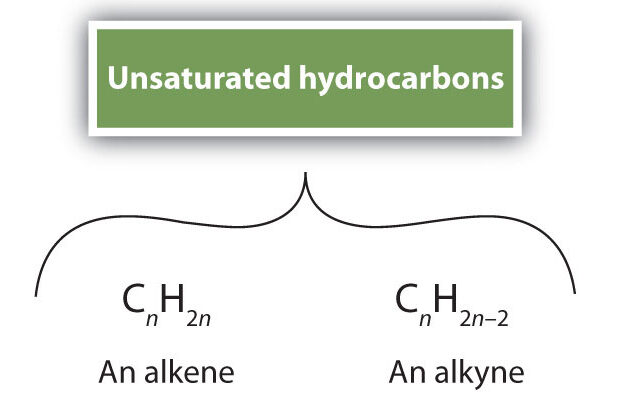
Explanation:
- Unsaturated compounds contain double (C=C) or triple (C≡C) bonds between carbon atoms.
- These multiple bonds can be broken to allow addition reactions — for example, with hydrogen or bromine.
- Unsaturated compounds are generally more reactive than saturated compounds.
- They belong mainly to the alkene (C=C) and alkyne (C≡C) series of hydrocarbons.
Examples:
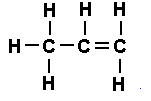
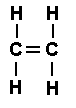
- Ethene (\(\mathrm{C_2H_4}\)) – contains a double bond (C=C).
- Propyne (\(\mathrm{C_3H_4}\)) – contains a triple bond (C≡C).
Displayed Formula of Ethene (\(\mathrm{C_2H_4}\)):
Key Idea: Unsaturated compounds have at least one C=C or C≡C bond, making them capable of addition reactions and more chemically reactive than saturated compounds.
Example :
Explain why ethene is described as an unsaturated compound.
▶️ Answer/Explanation
Step 1: Ethene (\(\mathrm{C_2H_4}\)) contains a carbon–carbon double bond.
Step 2: This means not all carbon–carbon bonds are single.
Final Answer: Ethene is unsaturated because it contains a C=C double bond.
Homologous Series
A homologous series is a family of similar compounds with similar chemical properties.
Explanation:
- All members of a homologous series have the same general formula.
- They show a gradual change in physical properties (e.g. boiling point, density) as molecular size increases.

- They have similar chemical properties because they contain the same functional group.
- Each successive member differs from the previous one by a CH₂ group.
Examples of Homologous Series:
- Alkanes: \(\mathrm{CH_4, C_2H_6, C_3H_8, C_4H_{10}}\)
- Alkenes: \(\mathrm{C_2H_4, C_3H_6, C_4H_8}\)
- Alcohols: \(\mathrm{CH_3OH, C_2H_5OH, C_3H_7OH}\)
Key Features of a Homologous Series:
- Same general formula.
- Same functional group → similar chemical reactions.
- Successive members differ by one \(\mathrm{CH_2}\) group.
- Gradual change in physical properties (e.g. increasing boiling points).
Example:
Alkane Series (General Formula: \(\mathrm{C_nH_{2n+2}}\))
Key Idea: All members of a homologous series react in a similar way because they have the same functional group.
Example :
State two features that all members of a homologous series have in common.
▶️ Answer/Explanation
Step 1: They all have the same functional group, so they have similar chemical properties.
Step 2: Each successive member differs by a CH₂ unit.
Final Answer: Same functional group and differ by one CH₂ group.
General Characteristics of a Homologous Series
A homologous series is a family of compounds with similar chemical properties and a gradual change in physical properties.
(a) Same General Formula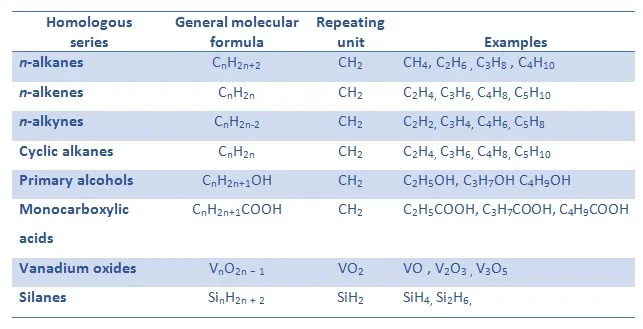
- All members of a homologous series share the same general formula.
- The general formula shows the relationship between the number of carbon (C) and hydrogen (H) atoms in each molecule.
- Each successive compound differs by one CH₂ group.
- This gives rise to a regular pattern in molecular structure.
Examples:
- Alkanes → \(\mathrm{C_nH_{2n+2}}\)
- Alkenes → \(\mathrm{C_nH_{2n}}\)
- Alcohols → \(\mathrm{C_nH_{2n+2}O}\)
(b) Trend in Physical Properties
Members of a homologous series show a gradual change in physical properties as molecular size (or relative molecular mass) increases.
- For example, as the number of carbon atoms increases:
- Boiling point increases
- Melting point increases
- Volatility decreases
- These changes occur because the strength of the intermolecular forces increases with larger molecules.
Example: Alkanes
| Alkane | Formula | Boiling Point (°C) | State at Room Temperature |
|---|---|---|---|
| Methane | CH₄ | -164 | Gas |
| Ethane | C₂H₆ | -89 | Gas |
| Propane | C₃H₈ | -42 | Gas |
| Butane | C₄H₁₀ | 0 | Gas/Liquid |
| Pentane | C₅H₁₂ | 36 | Liquid |
Key Idea: Members of a homologous series have the same general formula, the same functional group, and show a gradual trend in physical properties as molecular size increases.
Example :
Explain why the boiling point of alkanes increases as the number of carbon atoms increases.
▶️ Answer/Explanation
Step 1: As the number of carbon atoms increases, the alkane molecules become larger.
Step 2: The strength of the intermolecular (van der Waals) forces increases.
Final Answer: More energy is needed to break these forces, so the boiling point increases with molecular size.
