CIE iGCSE Co-ordinated Sciences-C11.3 Fuels- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-C11.3 Fuels – Study Notes
CIE iGCSE Co-ordinated Sciences-C11.3 Fuels – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
- Name the fossil fuels: coal, natural gas and petroleum
- Name methane as the main constituent of natural gas
- State that hydrocarbons are compounds that contain hydrogen and carbon only
- State that petroleum is a mixture of hydrocarbons
- Describe the separation of petroleum into useful fractions by fractional distillation
- Name the uses of the fractions as:
(a) refinery gas fraction for gas used in heating and cooking
(b) gasoline / petrol fraction for fuel used in cars
(c) naphtha fraction as a chemical feedstock
(d) diesel oil / gas oil for fuel used in diesel engines
(e) bitumen for making roads
Supplement
- Describe how the properties of fractions obtained from petroleum change from the bottom to the top of the fractionating column, limited to:
(a) decreasing chain length
(b) lower boiling points
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Fossil Fuels
- Fossil fuels are natural fuels formed from the remains of ancient plants and animals buried underground for millions of years.

- They are rich in carbon and release energy when burned.
Main Fossil Fuels:
- Coal – A solid fossil fuel formed from ancient plant material buried and compressed over millions of years.
- Natural Gas – A gaseous fossil fuel mainly composed of methane (\(\mathrm{CH_4}\)).
- Petroleum (Crude Oil) – A liquid fossil fuel formed from the remains of marine organisms; a mixture of many hydrocarbons.
Key Idea:
- All fossil fuels are non-renewable energy sources — they take millions of years to form and are being used up faster than they are created.
- They are mainly composed of hydrocarbons (compounds of hydrogen and carbon).
Example :
Name three fossil fuels commonly used as energy sources.
▶️ Answer/Explanation
Step 1: Fossil fuels include solid, liquid, and gaseous types.
Step 2: Examples: Coal (solid), Petroleum (liquid), Natural Gas (gas).
Final Answer: The three main fossil fuels are coal, natural gas, and petroleum.
Main Constituent of Natural Gas
The main constituent of natural gas is methane (\(\mathrm{CH_4}\)).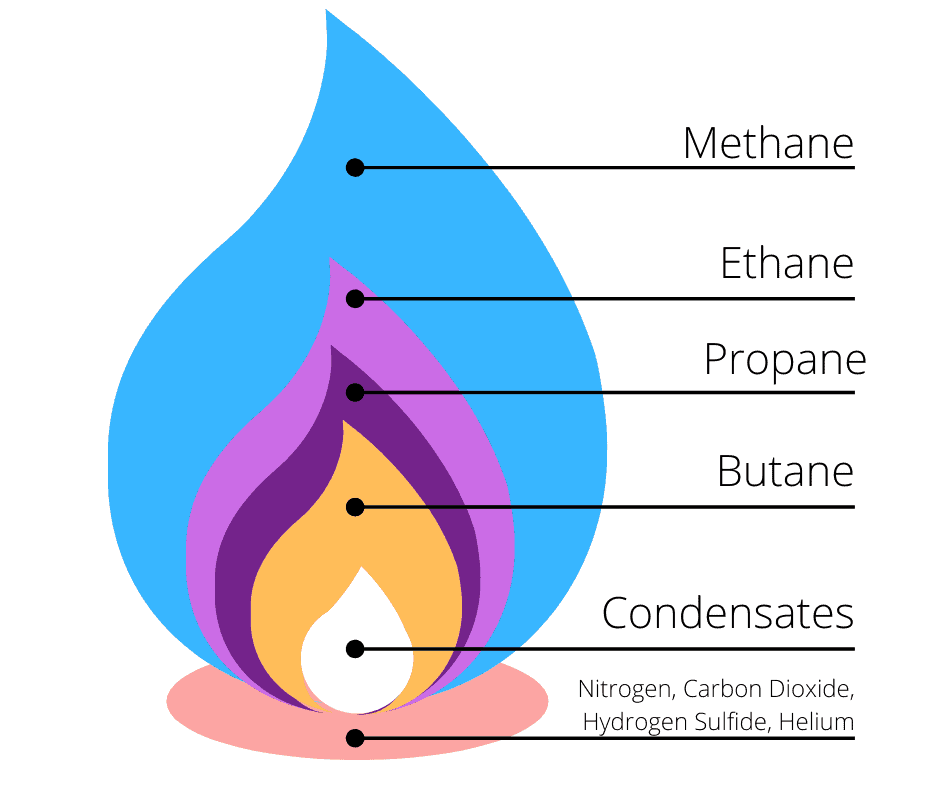
Explanation:
- Natural gas is a fossil fuel formed from the remains of microscopic marine organisms buried millions of years ago.
- It is a mixture of hydrocarbons, but contains mainly methane.
- Other minor components may include ethane (\(\mathrm{C_2H_6}\)), propane (\(\mathrm{C_3H_8}\)), and butane (\(\mathrm{C_4H_{10}}\)).
Chemical Formula of Methane:
\(\mathrm{CH_4}\)
Key Idea: Natural gas is mainly composed of methane, a simple hydrocarbon that burns cleanly to produce carbon dioxide and water.
Example :
What is the main component of natural gas?
▶️ Answer/Explanation
Step 1: Natural gas is a mixture of hydrocarbons.
Step 2: The major hydrocarbon present is methane.
Final Answer: The main constituent of natural gas is methane (\(\mathrm{CH_4}\)).
Hydrocarbons
Hydrocarbons are compounds that contain hydrogen and carbon only.![]()
Explanation:
- They are made up exclusively of two elements: hydrogen (H) and carbon (C).
- Hydrocarbons are the main constituents of fossil fuels such as natural gas, petroleum, and coal.
- They can be classified as:
- Alkanes – saturated hydrocarbons with only single C–C bonds.
- Alkenes – unsaturated hydrocarbons with at least one C=C double bond.
- Alkynes (beyond GCSE) – contain a C≡C triple bond.
Examples of Hydrocarbons:
- Methane (\(\mathrm{CH_4}\))
- Ethane (\(\mathrm{C_2H_6}\))
- Ethene (\(\mathrm{C_2H_4}\))
- Propane (\(\mathrm{C_3H_8}\))
Key Idea: All hydrocarbons contain only hydrogen and carbon atoms — no oxygen, nitrogen, or other elements are present.
Example :
What elements are present in all hydrocarbons?
▶️ Answer/Explanation
Step 1: Hydrocarbons are compounds composed of only two elements.
Step 2: These elements are hydrogen (H) and carbon (C).
Final Answer: All hydrocarbons contain hydrogen and carbon only.
Petroleum (Crude Oil)
Petroleum, also called crude oil, is a mixture of hydrocarbons.![]()
Explanation:
- Petroleum is formed naturally over millions of years from the remains of marine organisms buried under sedimentary rock.
- It contains a large variety of hydrocarbons — compounds made up only of hydrogen and carbon atoms.
- These hydrocarbons have different chain lengths and boiling points, which is why petroleum can be separated into useful parts by fractional distillation.
Examples of Hydrocarbons Found in Petroleum:
- Alkanes: Methane (\(\mathrm{CH_4}\)), Ethane (\(\mathrm{C_2H_6}\)), Propane (\(\mathrm{C_3H_8}\)), Octane (\(\mathrm{C_8H_{18}}\))
- Alkenes: Ethene (\(\mathrm{C_2H_4}\)), Propene (\(\mathrm{C_3H_6}\))
Key Idea: Petroleum is not a single substance but a complex mixture of many hydrocarbons, mostly alkanes, with varying chain lengths (from very short gases to long-chain liquids and waxes).
Example :
What is petroleum made up of?
▶️ Answer/Explanation
Step 1: Petroleum is a natural fossil fuel.
Step 2: It contains many different hydrocarbons with varying molecular sizes.
Final Answer: Petroleum is a mixture of hydrocarbons.
Separation of Petroleum by Fractional Distillation
Fractional distillation is the process used to separate petroleum (crude oil) into its useful components (fractions) based on their different boiling points.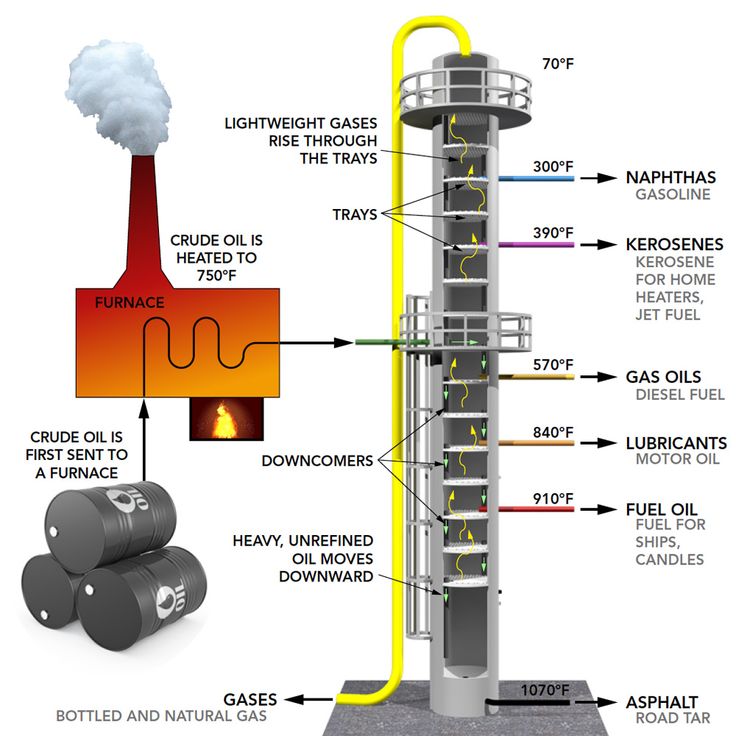
Explanation of the Process:
- Heating: Crude oil is heated in a furnace until most of it vaporises.
- Entering the fractionating column: The vapour mixture enters the column near the bottom. The column is hotter at the base and cooler at the top.
- Condensation: Different hydrocarbons condense at different levels in the column depending on their boiling points:
- High-boiling-point hydrocarbons condense near the bottom (where it is hot).
- Low-boiling-point hydrocarbons rise higher and condense near the top (where it is cooler).
- Collection: The condensed fractions are collected at various levels in the column.
Key Idea:
- Each fraction contains hydrocarbons with a similar range of boiling points.
- Fractions are used for different purposes depending on their physical properties (e.g. viscosity, flammability).
Major Fractions from Petroleum and Their Uses:
| Fraction | Approx. Boiling Range (°C) | Main Hydrocarbons | Uses |
|---|---|---|---|
| Refinery Gas | Below 40°C | C₁–C₄ (e.g. methane, ethane) | Fuel for heating and cooking; LPG |
| Petrol (Gasoline) | 40–100°C | C₅–C₉ | Fuel for cars |
| Kerosene | 150–250°C | C₁₀–C₁₆ | Fuel for jet aircraft and lamps |
| Diesel Oil | 250–350°C | C₁₄–C₂₀ | Fuel for diesel engines and heavy vehicles |
| Lubricating Oil / Fuel Oil | 350–500°C | C₂₀–C₄₀ | Lubricants, ships’ fuel, heating oil |
| Bitumen (Residue) | Above 500°C | Very large molecules | Road surfacing, roofing |
Temperature Gradient in the Fractionating Column:
- Hot at the bottom → cool at the top.
- Small molecules → low boiling points → collected at the top.
- Large molecules → high boiling points → collected near the bottom.
Key Idea: Fractional distillation separates crude oil into fractions with similar boiling points and molecular sizes, allowing each fraction to be used for a specific purpose.
Example :
Explain why the refinery gas fraction collects at the top of the fractionating column.
▶️ Answer/Explanation
Step 1: Refinery gas contains small hydrocarbon molecules.
Step 2: Small molecules have very low boiling points.
Step 3: They remain as gases and rise to the cooler top of the column.
Final Answer: Refinery gas collects at the top because it has the lowest boiling point.
Uses of Petroleum Fractions
Each fraction obtained from the fractional distillation of petroleum (crude oil) has a specific range of boiling points and is used for different purposes depending on its physical properties.
![]()
Main Fractions and Their Uses:
| Fraction | Main Use | Explanation |
|---|---|---|
| (a) Refinery Gas | Used as fuel for heating and cooking | A mixture of small gaseous hydrocarbons (mainly propane and butane) with very low boiling points; sold as LPG (liquefied petroleum gas). |
| (b) Gasoline / Petrol | Used as fuel for cars | Contains hydrocarbons with 5–9 carbon atoms; volatile and burns easily to power car engines. |
| (c) Naphtha | Used as a chemical feedstock | Used in the petrochemical industry to make plastics, detergents, and other organic chemicals. |
| (d) Diesel Oil / Gas Oil | Used as fuel for diesel engines | Contains larger hydrocarbon molecules; used in buses, lorries, and some cars. |
| (e) Bitumen (Residue) | Used for road surfacing and roofing | Thick, sticky material left at the bottom of the distillation column; used for waterproofing and making roads. |
Key Idea:
- The boiling point and size of hydrocarbon molecules determine each fraction’s physical properties and uses.
- Smaller molecules → more flammable → used as fuels.
- Larger molecules → thicker and less volatile → used for lubrication or road surfacing.
Example :
State the use of the bitumen fraction obtained from crude oil.
▶️ Answer/Explanation
Step 1: Bitumen is the residue left at the bottom of the fractionating column.
Step 2: It is a thick, sticky material with a very high boiling point.
Final Answer: Bitumen is used for road surfacing and roofing.
