CIE iGCSE Co-ordinated Sciences-C11.7 Polymers- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-C11.7 Polymers – Study Notes
CIE iGCSE Co-ordinated Sciences-C11.7 Polymers – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
- Define polymers as large molecules built up from many smaller molecules called monomers
- Describe the formation of poly(ethene) as an example of addition polymerisation using ethene monomers
- Identify the repeat units in addition polymers and in condensation polymers
- Deduce the structure or repeat unit of an addition polymer from a given alkene and vice versa
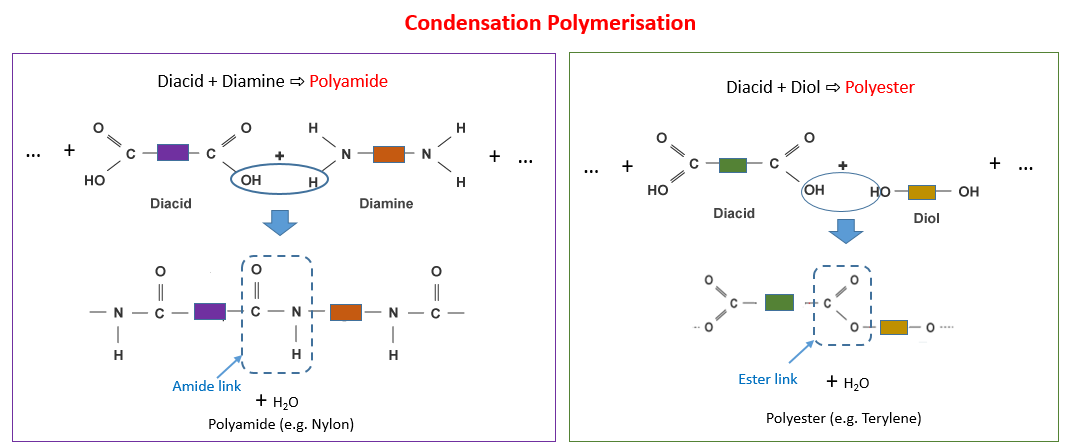
- Describe the differences between addition and condensation polymerisation
- Describe and draw the structure of nylon, a polyamide

CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Definition of Polymers and Monomers
Polymers are large molecules made up of many smaller, repeating units called monomers. Each monomer joins together in a repeating pattern to form a long-chain molecule (the polymer).
Explanation:
- Polymers are formed in a process called polymerisation.
- The small molecules (monomers) have bonds that allow them to link together repeatedly.
- The resulting polymer has very high molecular mass compared to the monomer.
Key Terms:
- Monomer: A small molecule that can join with other similar molecules to form a polymer.
- Polymer: A large molecule made by joining together many monomers.
- Polymerisation: The chemical process of joining monomers to form a polymer.
Example — Ethene to Poly(ethene)
- Monomer: Ethene (\(\mathrm{C_2H_4}\))
- Polymer: Poly(ethene) (\(\mathrm{(C_2H_4)_n}\))
Word Summary:
Many ethene molecules → Poly(ethene)
Chemical Equation:
\(\mathrm{nCH_2=CH_2 → (–CH_2–CH_2–)_n}\)
Everyday Examples of Polymers:
- Poly(ethene): plastic bags, bottles.
- Poly(propene): ropes, carpets.
- Poly(chloroethene) / PVC: water pipes, insulation.
- Poly(tetrafluoroethene) / PTFE: non-stick cookware coating.
Polymers are long-chain molecules made from the repetition of monomers. Each monomer contributes part of the repeating unit in the polymer chain.
Example :
Define a polymer and give one example of a polymer and its monomer.
▶️ Answer/Explanation
Step 1: A polymer is a large molecule made by joining many small molecules (monomers) together.
Step 2: Example — Poly(ethene) is made from many ethene monomers.
Final Answer: A polymer is a large molecule built from many smaller monomers; e.g. \(\mathrm{nCH_2=CH_2 → (–CH_2–CH_2–)_n}\).
Formation of Poly(ethene) — Example of Addition Polymerisation
Addition polymerisation is a chemical reaction in which many small alkene molecules (monomers) join together to form a large molecule called a polymer. No other product is formed — the polymer is the only product.
Example: Ethene → Poly(ethene)![]()
- Monomer: Ethene (\(\mathrm{CH_2=CH_2}\)) — an alkene with a double bond.
- Polymer: Poly(ethene) (\(\mathrm{(–CH_2–CH_2–)_n}\)) — a long-chain saturated molecule.
Word Equation:
Many ethene molecules → Poly(ethene)
Chemical Equation:
\(\mathrm{nCH_2=CH_2 → (–CH_2–CH_2–)_n}\)
Explanation:
- The double bond in each ethene molecule opens up (breaks) during the reaction.
- Each monomer then links to its neighbours, forming a long chain of repeating units.
- The resulting polymer (poly(ethene)) contains only single bonds between carbon atoms, making it a saturated compound.
Conditions for Polymerisation:
- High temperature and pressure.
- Catalyst (e.g. oxygen or peroxide catalyst) to initiate the reaction.
Properties of Poly(ethene):
- Lightweight and flexible.
- Chemically unreactive and waterproof.
- Used widely in packaging materials, plastic bags, and bottles.
In addition polymerisation, alkenes (like ethene) act as monomers. The double bonds open up and join together, forming a long chain polymer — poly(ethene) — with no other products.
| Term | Explanation | Example (Ethene → Poly(ethene)) |
|---|---|---|
| Monomer | Small molecule with a C=C double bond. | Ethene (\(\mathrm{CH_2=CH_2}\)) |
| Polymer | Large molecule formed by joining many monomers. | Poly(ethene) (\(\mathrm{(–CH_2–CH_2–)_n}\)) |
| Type of Reaction | Addition polymerisation (no by-products). | Ethene → Poly(ethene) |
Example :
Describe how poly(ethene) is formed from ethene monomers and write a chemical equation for the reaction.
▶️ Answer/Explanation
Step 1: Poly(ethene) is formed when many ethene molecules react together under high temperature and pressure, with a catalyst.
Step 2: The double bond in each ethene molecule breaks open, allowing them to link together in a chain.
Step 3: Equation — \(\mathrm{nCH_2=CH_2 → (–CH_2–CH_2–)_n}\)
Final Answer: Poly(ethene) is formed by addition polymerisation of ethene molecules, producing a long-chain polymer with no other products.
Identifying Repeat Units in Polymers
A repeat unit is the smallest group of atoms that repeats itself to form the polymer chain. In polymer structures, the repeat unit shows how the monomers are linked together.
Repeat Units in Addition Polymers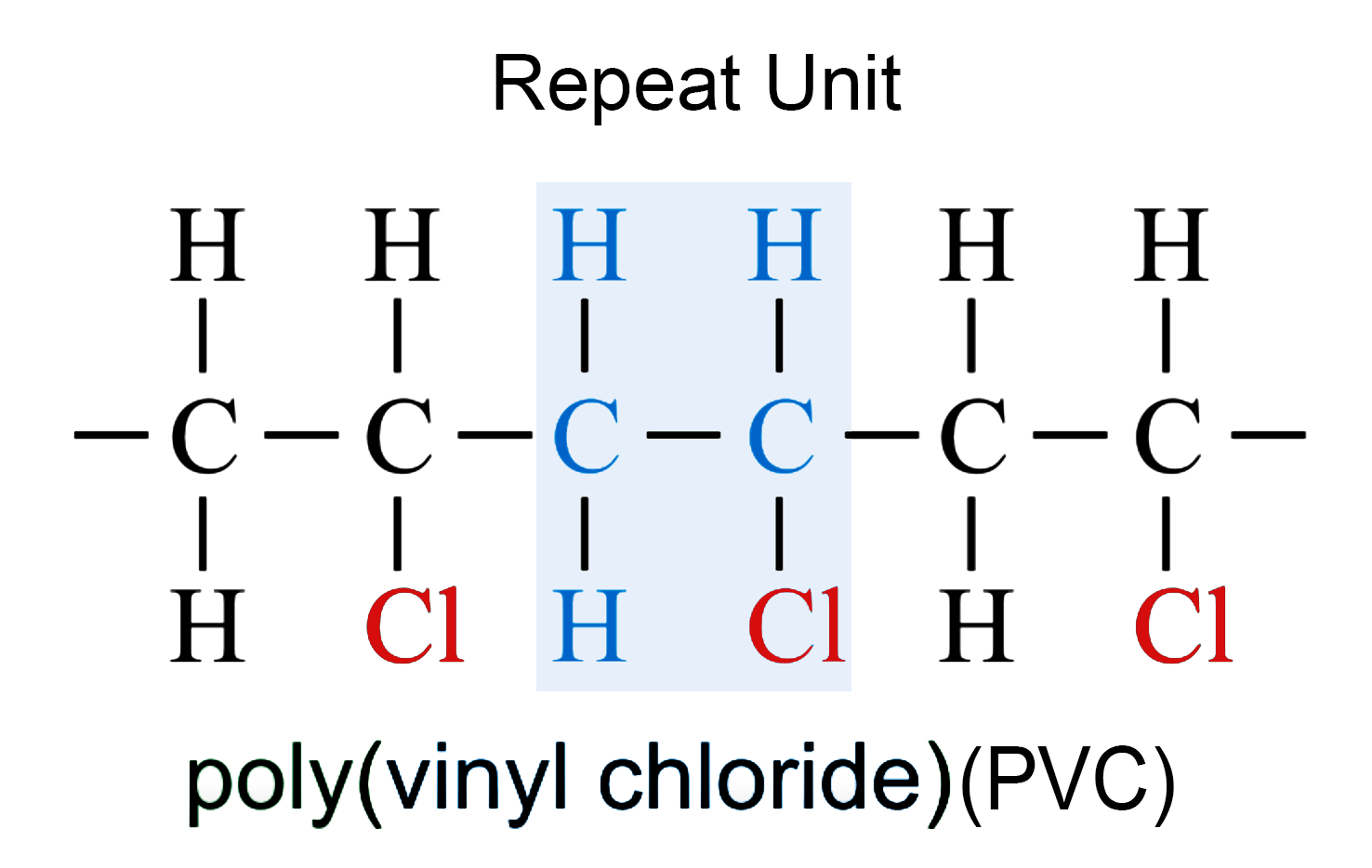
- Addition polymers are formed when alkene monomers (with C=C double bonds) join together.
- In the polymer, the double bond breaks and the monomers link together in a continuous chain of single bonds.
- The repeat unit is derived directly from the monomer — it has the same atoms, but with the C=C replaced by single C–C bonds.
Example: Poly(ethene)
- Monomer: Ethene (\(\mathrm{CH_2=CH_2}\))
- Polymer: Poly(ethene) (\(\mathrm{(–CH_2–CH_2–)_n}\))
Key Point: For addition polymers, the repeat unit is the same as the monomer but with the double bond replaced by single bonds.
Other Examples of Addition Polymers:
| Monomer | Polymer | Repeat Unit |
|---|---|---|
| Ethene (\(\mathrm{CH_2=CH_2}\)) | Poly(ethene) | [–CH₂–CH₂–] |
| Propene (\(\mathrm{CH_2=CHCH_3}\)) | Poly(propene) | [–CH₂–CH(CH₃)–] |
| Chloroethene (\(\mathrm{CH_2=CHCl}\)) | Poly(chloroethene) (PVC) | [–CH₂–CHCl–] |
Repeat Units in Condensation Polymers
Condensation polymers are formed when two different monomers with two functional groups react together.
- Each time the monomers join, a small molecule (usually water or HCl) is produced.
- The repeat unit in a condensation polymer contains atoms from both monomers but does not include the atoms of the small molecule lost.
Example: Formation of a Polyester (from a diacid and a diol)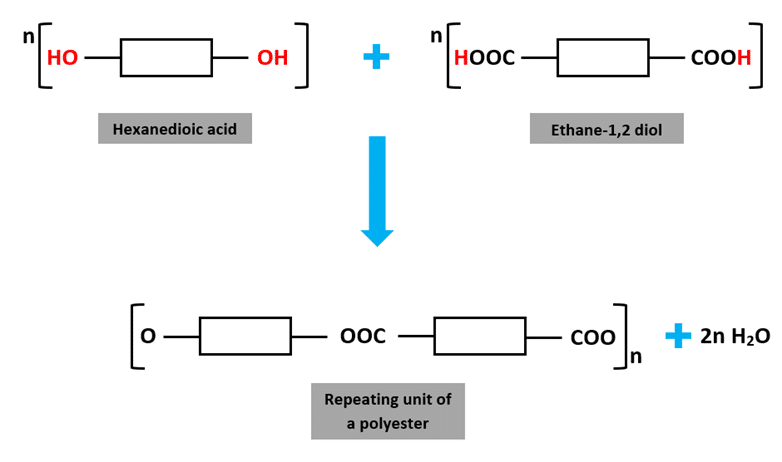
Monomers:
- Ethane-1,2-diol (\(\mathrm{HO–CH_2–CH_2–OH}\))
- Benzenedicarboxylic acid (\(\mathrm{HOOC–C_6H_4–COOH}\))
Reaction:
\(\mathrm{nHO–CH_2–CH_2–OH + nHOOC–C_6H_4–COOH → [–O–CH_2–CH_2–OOC–C_6H_4–CO–]_n + 2nH_2O}\)
Key Point: In condensation polymers, the repeat unit is made from parts of two different monomers, joined by new bonds (e.g., ester or amide links), and one small molecule (like water) is eliminated during each link formation.
| Type of Polymer | Monomers | Bonds Formed | By-products | Example Repeat Unit |
|---|---|---|---|---|
| Addition Polymer | One type of alkene monomer | C–C single bonds | None | [–CH₂–CH₂–] |
| Condensation Polymer | Two monomers with 2 functional groups | Ester or amide bonds | Water or HCl | [–O–CH₂–CH₂–OOC–C₆H₄–CO–] |
Example :
(a) Identify the repeat unit of poly(ethene).
(b) Identify the repeat unit in a polyester made from a diol and a dicarboxylic acid.
▶️ Answer/Explanation
(a) For poly(ethene): \(\mathrm{[–CH_2–CH_2–]}\)
(b) For polyester: \(\mathrm{[–O–CH_2–CH_2–OOC–C_6H_4–CO–]}\)
Step 1: The addition polymer repeat unit is identical to the monomer, except the double bond becomes a single bond.
Step 2: In a condensation polymer, the repeat unit combines atoms from both monomers, minus a small molecule (e.g., water).
Deduce the Repeat Unit of an Addition Polymer from an Alkene — and Vice Versa
Addition polymers form when an alkene monomer (contains C=C) opens its double bond and links in a chain.
To go from monomer → repeat unit: replace the C=C by single C–C and put the resulting unit in brackets.
To go from repeat unit → monomer: remove the brackets and change the single C–C back into a C=C, restoring the appropriate H (or substituent) counts.
Steps — Alkene → Repeat Unit![]()
- Write the alkene (show all atoms around the C=C if given).
- Break the double bond: replace = with – so each formerly double-bonded C now has one extra single bond to the neighbouring repeat unit.
- Enclose the resulting single-bonded fragment in square brackets with a subscript n to show repetition.
Steps — Repeat Unit → Alkene (monomer)
- Remove the brackets and the n.
- Change the single bond between the two carbon atoms of the repeat unit into a double bond (C=C).
- Adjust H (or substituent) counts so each carbon has four bonds total.
Worked Examples — Monomer → Repeat Unit
Ethene (CH₂=CH₂)
- Monomer: CH2 = CH2
- Repeat unit: [–CH2–CH2–]
- Polymer: (–CH2–CH2–)n
Propene (CH2=CH–CH3)
- Monomer: CH2 = CH – CH3
- Repeat unit: [–CH2–CH(CH3)–]
- Polymer: (–CH2–CH(CH3)–)n (poly(propene))
Chloroethene (CH2=CHCl) → PVC
- Monomer: CH2 = CHCl
- Repeat unit: [–CH2–CH(Cl)–]
- Polymer: (–CH2–CH(Cl)–)n (poly(chloroethene) / PVC)
Worked Examples — Repeat Unit → Monomer![]()
Given repeat unit: [–CH2–CH2–]
- Step 1: remove brackets → –CH2–CH2–
- Step 2: change single C–C to C=C → CH2=CH2
- Monomer: ethene
Given repeat unit: [–CH2–CH(Cl)–]
- Step 1: remove brackets → –CH2–CH(Cl)–
- Step 2: change single C–C to C=C → CH2=CHCl
- Monomer: chloroethene (vinyl chloride)
Example :
Deduce the repeat unit for the polymer formed from the alkene but-2-ene (\(\mathrm{CH_3–CH=CH–CH_3}\)).
▶️ Answer/Explanation
Step 1: Write the structure of the monomer:
\(\mathrm{CH_3–CH=CH–CH_3}\)
Step 2: During addition polymerisation, the C=C double bond opens up to form C–C single bonds, linking the monomers together.
Step 3: Each carbon keeps its attached group (–CH₃).
Step 4: The repeating unit of the polymer is written inside brackets with a subscript \( n \):
Monomer: CH3–CH=CH–CH3
Repeat unit: [–CH(CH3)–CH(CH3)–]
Polymer: (–CH(CH3)–CH(CH3)–)n
The repeat unit of the polymer formed from but-2-ene is [–CH(CH₃)–CH(CH₃)–].
Differences Between Addition and Condensation Polymerisation
Polymerisation is the process by which small molecules called monomers join together to form large molecules called polymers.
There are two main types of polymerisation: addition polymerisation and condensation polymerisation.
1. Addition Polymerisation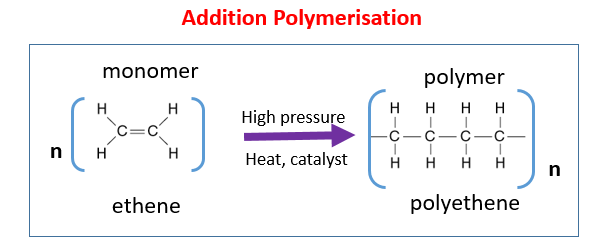
- Occurs when alkene monomers (with C=C double bonds) join together.
- The double bonds in the monomers open up to form single bonds linking them into a long chain.
- No other products are formed — the polymer is the only product.
Example:
\(\mathrm{nCH_2=CH_2 → (–CH_2–CH_2–)_n}\)
Ethene → Poly(ethene)
Features:
- Monomer must contain a C=C double bond.
- Only one product is formed (the polymer).
- No small molecules (like water or HCl) are released.
- Example polymers: poly(ethene), poly(propene), PVC, PTFE.
2. Condensation Polymerisation
- Occurs when two different monomers, each with two functional groups, react together.

- A small molecule (often water or hydrogen chloride) is released each time a bond forms.
- The polymer formed contains linkages such as ester (–COO–) or amide (–CONH–) bonds.
Example: Formation of a Polyester
\(\mathrm{nHO–CH_2–CH_2–OH + nHOOC–C_6H_4–COOH → [–O–CH_2–CH_2–OOC–C_6H_4–CO–]_n + 2nH_2O}\)
Ethane-1,2-diol + Terephthalic acid → Polyester (PET) + Water
Features:
- Monomers have two functional groups each (e.g. –COOH, –OH, –NH₂).
- Two products are formed: the polymer and a small molecule (usually water or HCl).
- Linkages such as ester or amide are formed between repeating units.
- Example polymers: nylon, polyester, proteins.
Addition vs Condensation Polymerisation
| Feature | Addition Polymerisation | Condensation Polymerisation |
|---|---|---|
| Type of monomer | Alkene monomers with C=C bonds | Monomers with two functional groups (e.g. –COOH, –OH, –NH₂) |
| Type of reaction | Addition (C=C bond opens and joins) | Condensation (bonds form with elimination of small molecules) |
| Products | Only one — the polymer | Two — the polymer and a small molecule (e.g. water, HCl) |
| Bonds formed | C–C single bonds | Ester (–COO–) or Amide (–CONH–) bonds |
| By-product | None | Water or hydrogen chloride |
| Examples | Poly(ethene), Poly(propene), PVC | Nylon, Polyester (PET), Proteins |
Key Idea:
- In addition polymerisation — the polymer is the only product (from alkenes).
- In condensation polymerisation — each link formation releases a small molecule (like water or HCl).
Example :
State two differences between addition and condensation polymerisation, and give one example of each.
▶️ Answer/Explanation
Step 1: Addition polymerisation uses alkene monomers and produces only the polymer; condensation polymerisation uses monomers with two functional groups and releases small molecules such as water.
Step 2: Example of addition polymer: Poly(ethene). Example of condensation polymer: Nylon or polyester (PET).
Final Answer: Addition polymerisation involves alkenes only and produces no by-products, while condensation polymerisation involves two functional group monomers and releases a small molecule such as water.
Structure and Description of Nylon — A Polyamide
Nylon is a type of condensation polymer called a polyamide.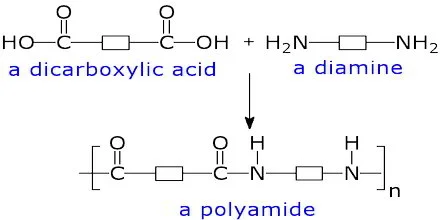
- It is formed when two different monomers, each containing two functional groups, react together and eliminate a small molecule (usually water).
- The key functional group formed in nylon is the amide linkage (\(\mathrm{–CONH–}\)).
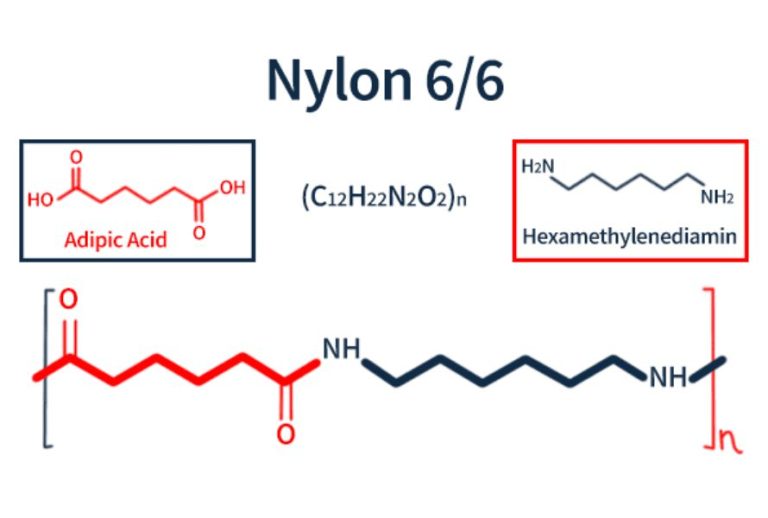
Formation of Nylon (Example: Nylon-6,6)
Monomers:
- 1,6-diaminohexane (\(\mathrm{H_2N–(CH_2)_6–NH_2}\))
- Hexanedioic acid (Adipic acid) (\(\mathrm{HOOC–(CH_2)_4–COOH}\))
Reaction:
- Each –NH₂ group from the diamine reacts with a –COOH group from the dicarboxylic acid.
- This forms an amide bond (\(\mathrm{–CONH–}\)) and eliminates a molecule of water (H₂O) for each link formed.
Condensation Reaction Equation:
\(\mathrm{nH_2N–(CH_2)_6–NH_2 + nHOOC–(CH_2)_4–COOH → [–NH–(CH_2)_6–NH–CO–(CH_2)_4–CO–]_n + 2nH_2O}\)
1,6-diaminohexane + hexanedioic acid → nylon-6,6 + water
Structure of Nylon (Polyamide):
This is the amide linkage (also called the peptide bond in proteins).
Key Features of Nylon:
- Formed by condensation polymerisation between a diamine and a dicarboxylic acid.
- Contains repeating amide bonds (–CONH–).
- Each linkage formation eliminates one molecule of water.
Properties of Nylon:
- Strong and durable due to hydrogen bonding between chains.
- High melting point.
- Resistant to wear, abrasion, and many chemicals.
- Used in clothing, ropes, parachutes, and machine parts.
| Monomer 1 | Monomer 2 | Linkage Formed | Small Molecule Released | Polymer Name |
|---|---|---|---|---|
| 1,6-diaminohexane | Hexanedioic acid | Amide (–CONH–) | Water (H₂O) | Nylon-6,6 |
Nylon is a polyamide made by the condensation polymerisation of a diamine and a dicarboxylic acid, forming strong –CONH– linkages and releasing water.
Example :
Describe how nylon-6,6 is formed and state the type of linkage it contains.
▶️ Answer/Explanation
Step 1: Nylon-6,6 is formed from 1,6-diaminohexane and hexanedioic acid by condensation polymerisation.
Step 2: Each –NH₂ group reacts with a –COOH group to form an amide linkage (–CONH–) and release water.
Step 3: The repeating unit of nylon-6,6 is: \(\mathrm{[–NH–(CH_2)_6–NH–CO–(CH_2)_4–CO–]_n}\)
Final Answer: Nylon-6,6 is a polyamide formed by condensation polymerisation, containing amide linkages (–CONH–) and releasing water as a by-product.