CIE iGCSE Co-ordinated Sciences-C12.5 Identification of ions and gases- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-C12.5 Identification of ions and gases – Study Notes
CIE iGCSE Co-ordinated Sciences-C12.5 Identification of ions and gases – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
- Describe tests to identify the anions:
(a) carbonate, CO₃²⁻, by reaction with dilute acid and then testing for carbon dioxide gas
(b) chloride, Cl⁻, bromide, Br⁻, and iodide, I⁻, by acidifying with dilute nitric acid then adding aqueous silver nitrate
(c) nitrate, NO₃⁻, reduction with aluminium foil and aqueous sodium hydroxide and then testing for ammonia gas
(d) sulfate, SO₄²⁻, by acidifying with dilute nitric acid and then adding aqueous barium nitrate - Describe tests using aqueous sodium hydroxide and aqueous ammonia to identify the aqueous cations:
(a) ammonium, NH₄⁺
(b) calcium, Ca²⁺
(c) copper(II), Cu²⁺
(d) iron(II), Fe²⁺
(e) iron(III), Fe³⁺
(f) zinc, Zn²⁺ (formulas of complex ions are not required) - Describe tests to identify the gases:
(a) ammonia, NH₃, using damp red litmus paper
(b) carbon dioxide, CO₂, using limewater
(c) chlorine, Cl₂, using damp litmus paper
(d) hydrogen, H₂, using a lighted splint
(e) oxygen, O₂, using a glowing splint - Describe the use of a flame test to identify the cations:
(a) lithium, Li⁺
(b) sodium, Na⁺
(c) potassium, K⁺
(d) copper(II), Cu²⁺
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Tests for Anions
Chemical tests are used to identify negative ions (anions) in unknown compounds.
(a) Carbonate (\(\mathrm{CO_3^{2-}}\))
- Add dilute acid (e.g. hydrochloric acid) to the sample.
- Carbon dioxide gas is produced if carbonate ions are present.
- Test the gas by bubbling it through limewater; it turns milky/cloudy.
\(\mathrm{CO_3^{2-} + 2H^+ \;\;\rightarrow\;\; CO_2 + H_2O}\)
(b) Halides: Chloride (\(\mathrm{Cl^-}\)), Bromide (\(\mathrm{Br^-}\)), Iodide (\(\mathrm{I^-}\))
- Acidify the solution with dilute nitric acid (to remove carbonates that interfere).
- Add aqueous silver nitrate (\(\mathrm{AgNO_3}\)).
- Observe the precipitate formed:
- Chloride (\(\mathrm{Cl^-}\)) → white precipitate (\(\mathrm{AgCl}\)).
- Bromide (\(\mathrm{Br^-}\)) → cream precipitate (\(\mathrm{AgBr}\)).
- Iodide (\(\mathrm{I^-}\)) → yellow precipitate (\(\mathrm{AgI}\)).
\(\mathrm{Ag^+ + Cl^- \;\;\rightarrow\;\; AgCl (s)}\)
(c) Nitrate (\(\mathrm{NO_3^-}\))
- Add aluminium foil and aqueous sodium hydroxide to the sample.
- Warm the mixture gently.
- Nitrate ions are reduced to ammonia gas, which turns damp red litmus paper blue.
\(\mathrm{NO_3^- + 4Al + 5OH^- \;\;\rightarrow\;\; NH_3 + 2H_2O + AlO_2^-}\)
(d) Sulfate (\(\mathrm{SO_4^{2-}}\))
![]()
- Acidify the solution with dilute nitric acid (to remove carbonates and sulfites).
- Add aqueous barium nitrate (\(\mathrm{Ba(NO_3)_2}\)).
- A white precipitate of barium sulfate (\(\mathrm{BaSO_4}\)) confirms the presence of sulfate ions.
\(\mathrm{SO_4^{2-} + Ba^{2+} \;\;\rightarrow\;\; BaSO_4 (s)}\)
Example :
A solution gives a white precipitate with silver nitrate after acidifying with nitric acid. Which ion is present?
▶️ Answer/Explanation
Step 1: Acidification with nitric acid removes carbonate interference.
Step 2: A white precipitate with silver nitrate corresponds to \(\mathrm{AgCl}\).
Final Answer: The ion present is chloride (\(\mathrm{Cl^-}\)).
Tests for Aqueous Cations using Sodium Hydroxide (NaOH) and Aqueous Ammonia (NH₃)
Cations can be identified by adding aqueous sodium hydroxide or aqueous ammonia and observing the precipitate formed or gas released.
(a) Ammonium (\(\mathrm{NH_4^+}\))
- Add aqueous sodium hydroxide and warm the mixture gently.
- Observation: Ammonia gas (\(\mathrm{NH_3}\)) is released → turns damp red litmus paper blue.

- No precipitate forms because ammonium salts are soluble.
(b) Calcium (\(\mathrm{Ca^{2+}}\))
- Add a few drops of aqueous sodium hydroxide.
- Observation with NaOH: White precipitate of \(\mathrm{Ca(OH)_2}\), insoluble in excess.
- Observation with NH₃: Very slight/no precipitate (calcium hydroxide is only sparingly soluble).
(c) Copper(II) (\(\mathrm{Cu^{2+}}\))
- With NaOH: Blue precipitate of \(\mathrm{Cu(OH)_2}\), insoluble in excess.
- With NH₃: Blue precipitate forms, dissolves in excess to give a deep blue solution.
(d) Iron(II) (\(\mathrm{Fe^{2+}}\))
- With NaOH: Green precipitate of \(\mathrm{Fe(OH)_2}\), turns brown on standing (oxidises to \(\mathrm{Fe(OH)_3}\)).
- With NH₃: Same result: green precipitate, turning brown on standing.
(e) Iron(III) (\(\mathrm{Fe^{3+}}\))
- With NaOH: Red-brown precipitate of \(\mathrm{Fe(OH)_3}\), insoluble in excess.
- With NH₃: Same result: red-brown precipitate, insoluble in excess.
(f) Zinc (\(\mathrm{Zn^{2+}}\))
- With NaOH: White precipitate of \(\mathrm{Zn(OH)_2}\), dissolves in excess to give a colourless solution.
- With NH₃: White precipitate, dissolves in excess to give a colourless solution.
Example :
A student added aqueous sodium hydroxide to an unknown solution. A green precipitate formed, which turned brown on standing. Identify the cation present.
▶️ Answer/Explanation
Step 1: A green precipitate with NaOH indicates \(\mathrm{Fe^{2+}}\).
Step 2: The brown colour on standing confirms oxidation of \(\mathrm{Fe^{2+}}\) to \(\mathrm{Fe^{3+}}\).
Final Answer: The cation is iron(II), \(\mathrm{Fe^{2+}}\).
Tests to Identify Gases
Chemical tests can be used to identify common gases by their characteristic reactions.
(a) Ammonia (\(\mathrm{NH_3}\))
![]()
- Test with damp red litmus paper.
- Observation: Turns damp red litmus paper blue (alkaline gas).
- Extra test: Has a strong pungent smell.
(b) Carbon Dioxide (\(\mathrm{CO_2}\))
![]()
- Bubble the gas through limewater (aqueous calcium hydroxide).
- Observation: Limewater turns milky/cloudy due to formation of calcium carbonate.
\(\mathrm{Ca(OH)_2 + CO_2 \;\;\rightarrow\;\; CaCO_3 + H_2O}\)
(c) Chlorine (\(\mathrm{Cl_2}\))
![]()
- Test with damp litmus paper.
- Observation: Litmus paper is first bleached white (regardless of starting colour).
(d) Hydrogen (\(\mathrm{H_2}\))
![]()
- Test with a lighted splint.
- Observation: Produces a characteristic ‘squeaky pop’ sound as it burns.
(e) Oxygen (\(\mathrm{O_2}\))
![]()
- Test with a glowing splint.
- Observation: The splint relights in oxygen.
Example :
A gas is tested with a glowing splint, which relights. Identify the gas and explain the observation.
▶️ Answer/Explanation
Step 1: A glowing splint relighting is the test for oxygen.
Step 2: Oxygen supports combustion strongly, causing the splint to relight.
Final Answer: The gas is oxygen (\(\mathrm{O_2}\)).
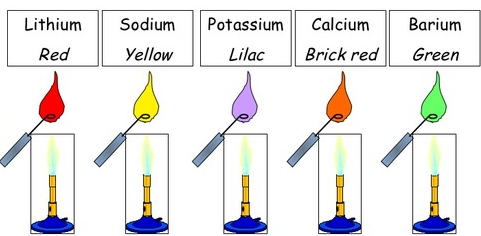
Flame Tests for Metal Cations
A flame test is used to identify certain metal cations by the characteristic colour they produce in a flame.
Method:
- Dip a clean wire loop (usually platinum or nichrome) into concentrated hydrochloric acid, then into the solid sample or solution of the metal compound.
- Place the loop in a non-luminous Bunsen burner flame.
- Observe the characteristic flame colour.

Flame Colours:
- (a) Lithium, \(\mathrm{Li^+}\): Red flame (crimson red).
- (b) Sodium, \(\mathrm{Na^+}\): Yellow flame (intense yellow).
- (c) Potassium, \(\mathrm{K^+}\): Lilac flame (pale purple).
- (d) Copper(II), \(\mathrm{Cu^{2+}}\): Blue-green flame.
Key Note: Each metal ion produces a unique flame colour due to the excitation of its electrons by heat energy.
Example :
A student carried out a flame test on an unknown salt and observed a lilac flame. Identify the cation present.
▶️ Answer/Explanation
Step 1: Recall the characteristic flame colours: Li⁺ = red, Na⁺ = yellow, K⁺ = lilac, Cu²⁺ = blue-green.
Step 2: Lilac flame corresponds to potassium ions.
Final Answer: The cation present is potassium, \(\mathrm{K^+}\).