CIE iGCSE Co-ordinated Sciences-C2.1 Elements, compounds and mixtures- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-C2.1 Elements, compounds and mixtures – Study Notes
CIE iGCSE Co-ordinated Sciences-C2.1 Elements, compounds and mixtures – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
- Describe the differences between elements, compounds and mixtures
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Elements, compounds and mixtures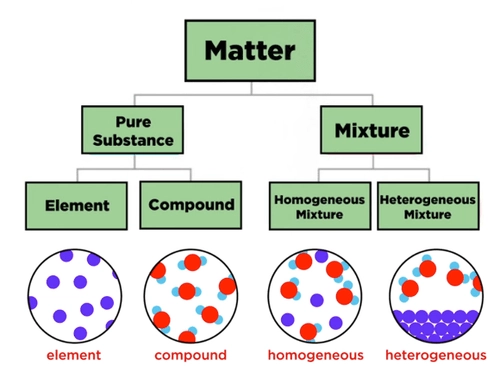
Elements:
- Pure substances made of only one type of atom.
- Cannot be broken down into simpler substances by chemical means.
Compounds:
- Pure substances made from two or more different elements chemically bonded in a fixed ratio.
- Can only be separated into elements by chemical reactions.
Mixtures:
- Contain two or more substances physically combined, not chemically bonded.
- Components can be separated by physical methods such as filtration, distillation, or chromatography.
| Property | Element | Compound | Mixture |
|---|---|---|---|
| Composition | Only one kind of atom | Two or more elements in a fixed ratio | Two or more substances; ratio not fixed |
| Separation | Cannot be separated chemically | Separated into elements by chemical reaction | Separated physically |
| Chemical bonding | No chemical bonds | Atoms chemically bonded | No chemical bonds between components |
| Properties | Uniform and characteristic of element | Different from the elements it contains | Combination of properties of components |
| Examples | \( \text{H}_2 \), \( \text{O}_2 \), \( \text{Au} \) | \( \text{H}_2\text{O} \), \( \text{CO}_2 \), \( \text{NaCl} \) | Air, saltwater, sand + iron filings |
Example
Classify the following substances as elements, compounds, or mixtures: oxygen gas, water, saltwater, gold, carbon dioxide.
▶️ Answer/Explanation
Oxygen gas (\( \text{O}_2 \)) – Element: contains only one type of atom.
Water (\( \text{H}_2\text{O} \)) – Compound: made of hydrogen and oxygen chemically bonded in a fixed ratio.
Saltwater – Mixture: contains water and dissolved salt physically combined; can be separated by evaporation.
Gold (\( \text{Au} \)) – Element: contains only gold atoms.
Carbon dioxide (\( \text{CO}_2 \)) – Compound: made of carbon and oxygen chemically bonded.
