CIE iGCSE Co-ordinated Sciences-C2.5 Simple molecules and covalent bonds- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-C2.5 Simple molecules and covalent bonds – Study Notes
CIE iGCSE Co-ordinated Sciences-C2.5 Simple molecules and covalent bonds – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
- State that a covalent bond is formed when a pair of electrons is shared between two atoms leading to noble gas electronic configurations
- Describe the formation of covalent bonds in simple molecules, including H₂, Cl₂, H₂O, CH₄, NH₃ and HCl. Use dot-and-cross diagrams to show the electronic configurations in these molecules
- Describe in terms of structure and bonding the properties of simple molecular compounds:
(a) low melting points and boiling points
(b) poor electrical conductivity
Supplement
- Describe the formation of covalent bonds in simple molecules, including CH₃OH, C₂H₄, O₂, CO₂ and N₂. Use dot-and-cross diagrams to show the electronic configurations in these molecules
- Explain in terms of structure and bonding the properties of simple molecular compounds:
(a) low melting points and boiling points in terms of weak intermolecular forces (specific types of intermolecular forces are not required)
(b) poor electrical conductivity
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Covalent Bonding
A covalent bond is formed when a pair of electrons is shared between two atoms. This sharing allows each atom to achieve a full outer electron shell, giving a noble gas electronic configuration.
Key Idea:![]()
- Covalent bonding usually occurs between non-metal atoms.
- Each atom contributes one (or more) electrons to the shared pair.
- The shared pair of electrons holds the atoms together in a molecule.
\(\mathrm{Covalent\ bond = Shared\ pair\ of\ electrons}\)
Why Covalent Bonds Form:
- Atoms share electrons so that each atom achieves a full outer shell.
- This results in a stable electron arrangement, similar to that of the nearest noble gas.
Example: Hydrogen atoms share electrons to get the same configuration as Helium.
Key Points on Covalent Bonding
| Feature | Description |
|---|---|
| Bond type | Covalent bond (shared electrons) |
| Occurs between | Non-metal atoms |
| Bond formation | Atoms share pairs of electrons |
| Resulting structure | Molecules (e.g. H2, O2, H2O) |
| Outer shell condition | Each atom achieves a full outer shell (noble gas configuration) |
Example Question :
Explain how a molecule of chlorine (Cl2) is formed in terms of covalent bonding.
▶️ Answer/Explanation
Step 1: Each chlorine atom has 7 electrons in its outer shell.
Step 2: They share one pair of electrons to achieve full outer shells.
Step 3: The shared pair forms a single covalent bond.
Final Answer: Each chlorine atom now has 8 outer electrons — a full noble gas configuration. The molecule formed is Cl2.
Formation of Covalent Bonds in Simple Molecules
A covalent bond is formed when two atoms share one or more pairs of electrons.
- Each atom achieves a full outer electron shell (a noble gas configuration).
- Covalent bonding usually occurs between non-metal atoms.
1. Hydrogen Molecule (H2)
![]()
- Each hydrogen atom has 1 electron.
- They share one pair of electrons → each atom now has 2 electrons (like helium).
Bond type: Single covalent bond (one shared pair of electrons).
2. Chlorine Molecule (Cl2)
![]()
- Each chlorine atom has 7 outer electrons.
- They share one pair of electrons so each has 8 electrons in the outer shell.
Bond type: Single covalent bond.
3. Hydrogen Chloride (HCl)
![]()
- Hydrogen (1 electron) shares one electron with chlorine (7 outer electrons).
- Hydrogen achieves 2 electrons; chlorine achieves 8 electrons.
Bond type: Single covalent bond.
4. Water Molecule (H2O)
![]()
- Oxygen has 6 outer electrons; hydrogen has 1 each.
- Oxygen shares one electron with each hydrogen atom.
- Each H gets 2 electrons (like He); O gets 8 electrons (like Ne).
Bond type: Two single covalent bonds (each O–H).
5. Methane (CH4)
![]()
- Carbon has 4 outer electrons; each hydrogen has 1.
- Carbon shares one electron with each of four hydrogen atoms → total of 4 shared pairs.
- Each atom achieves a full outer shell.
Bond type: Four single covalent bonds (C–H).
6. Ammonia (NH3)
![]()
- Nitrogen has 5 outer electrons; each hydrogen has 1.
- Nitrogen shares one electron with each hydrogen → 3 shared pairs total.
- Nitrogen achieves 8 electrons; each H gets 2.
Bond type: Three single covalent bonds (N–H).
| Molecule | Atoms Involved | Shared Electron Pairs | Bond Type |
|---|---|---|---|
| H2 | H–H | 1 | Single |
| Cl2 | Cl–Cl | 1 | Single |
| HCl | H–Cl | 1 | Single |
| H2O | H–O–H | 2 | Single |
| CH4 | C–H bonds | 4 | Single |
| NH3 | N–H bonds | 3 | Single |
Example :
Draw a dot-and-cross diagram for a molecule of methane (CH4).
▶️ Answer/Explanation
Step 1: Carbon has 4 outer electrons; hydrogen has 1 each.
Step 2: Carbon shares one electron with each hydrogen atom → 4 shared pairs total.
![]()
Step 3: Each H now has 2 electrons; C has 8 electrons → full shells.
Final Answer: Methane (CH4) has 4 single covalent bonds, each formed by a shared pair of electrons between C and H.
Properties of Simple Molecular Compounds
Simple molecular compounds are substances made up of small molecules containing atoms joined by strong covalent bonds inside each molecule. The molecules themselves are held together by weak intermolecular forces (forces between molecules).
Examples: \(\mathrm{H_2, O_2, H_2O, CH_4, CO_2, Cl_2}\)
Structure and Bonding:![]()
- Each molecule consists of atoms joined by strong covalent bonds (shared electrons).
- Between the molecules are weak intermolecular forces (also called Van der Waals forces).
- These weak forces determine many of their physical properties.
- Molecules → strong covalent bonds inside
- but weak forces between molecules
(a) Low Melting and Boiling Points
![]()
- Although covalent bonds inside molecules are strong, the forces between molecules (intermolecular forces) are weak.
- Only a small amount of energy is needed to overcome these weak forces.
- Therefore, simple molecular substances have low melting and boiling points.
- Most are gases or liquids at room temperature.
Example:
- Oxygen (O₂) and carbon dioxide (CO₂) are gases because their molecules are easily separated.
- Water (H₂O) has a higher melting/boiling point than expected due to hydrogen bonding, but still much lower than ionic compounds.
\(\mathrm{Weak\ intermolecular\ forces \Rightarrow\ low\ melting/boiling\ points}\)
(b) Poor Electrical Conductivity
![]()
- Simple molecular substances contain neutral molecules, not ions.
- There are no free electrons or ions to carry an electric current.
- Therefore, they are poor conductors of electricity — in both solid and liquid states.
Examples:
- Hydrogen (H₂), oxygen (O₂), methane (CH₄), and chlorine (Cl₂) do not conduct electricity.
- They are made of neutral molecules → no charged particles available for conduction.
\(\mathrm{No\ ions/electrons \Rightarrow\ no\ conduction\ of\ electricity}\)
Example :
Explain why oxygen (O₂) has a low boiling point and does not conduct electricity.
▶️ Answer/Explanation
Step 1: Oxygen is a simple molecular substance with O=O covalent bonds inside each molecule.
Step 2: The molecules are held together by weak intermolecular forces.
Step 3: These weak forces require little energy to overcome → low boiling point.
Step 4: Oxygen molecules contain no free electrons or ions → cannot conduct electricity.
Final Answer: Oxygen has a low boiling point because it has weak forces between molecules and it does not conduct electricity because it has no free charged particles.
Formation of Covalent Bonds in Simple Molecules (CH3OH, C2H4, O2, CO2, N2)
A covalent bond is formed when two atoms share one or more pairs of electrons. This sharing allows each atom to achieve a full outer shell of electrons — a noble gas configuration. These bonds occur between non-metal atoms.
1. Methanol (CH3OH)
![]()
- Carbon has 4 outer electrons, oxygen has 6, and hydrogen has 1 each.
- Carbon forms 4 single covalent bonds: 3 with H and 1 with O.
- Oxygen forms 2 single bonds: one with C and one with H.
- Each atom attains a full outer shell.
Bond types: 5 single covalent bonds (C–H ×3, C–O, O–H)
2. Ethene (C2H4)
![]()
- Each carbon atom has 4 outer electrons.
- Each carbon forms 3 single bonds (2 with H and 1 with the other carbon) and shares one more pair → a double bond (C=C).
Bond types: 1 double bond (C=C) and 4 single bonds (C–H)
3. Oxygen (O2)
![]()
- Each oxygen atom has 6 outer electrons.
- They share 2 pairs of electrons → a double bond (O=O).
- Each oxygen then has a full outer shell of 8 electrons.
Bond type: Double covalent bond (2 shared pairs)
4. Carbon Dioxide (CO2)
![]()
- Carbon has 4 outer electrons; each oxygen has 6.
- Carbon forms two double bonds, one with each oxygen atom.
- Each atom achieves a full outer shell.
Bond type: Two double covalent bonds (C=O ×2)
5. Nitrogen (N2)
![]()
- Each nitrogen atom has 5 outer electrons.
- They share 3 pairs of electrons → forming a triple bond (N≡N).
- This gives both nitrogen atoms a full outer shell (8 electrons).
Bond type: Triple covalent bond (3 shared pairs)
Example :
Draw a dot-and-cross diagram for carbon dioxide (CO2).
▶️ Answer/Explanation
Step 1: Carbon has 4 outer electrons, each oxygen has 6.
Step 2: Carbon forms double covalent bonds with both oxygens.
Step 3: Each atom now has a full outer shell (8 electrons).
![]()
Final Answer: Carbon dioxide has two double covalent bonds (C=O ×2), allowing all atoms to achieve full outer shells.
Properties of Simple Molecular Compounds (Structure and Bonding Explanation)
Simple molecular compounds are substances made up of small molecules joined by strong covalent bonds inside each molecule.
- These molecules are held together by weak intermolecular forces (forces between separate molecules).
- Their properties are determined by the strength of these weak forces, not by the strong covalent bonds within the molecules.
(a) Low Melting Points and Boiling Points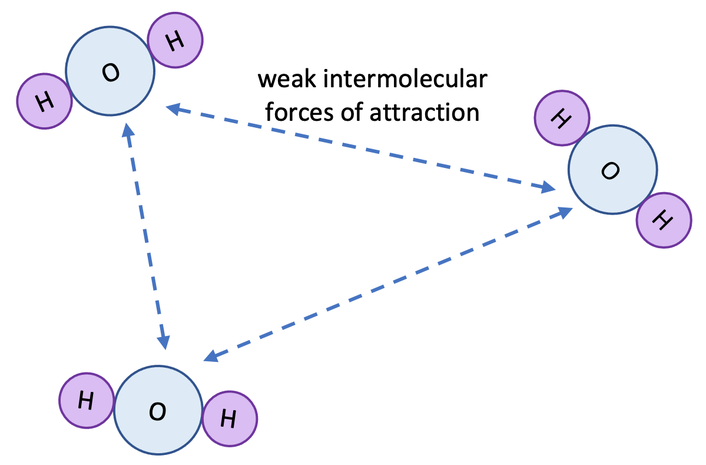
- Inside each molecule, atoms are bonded by strong covalent bonds.
- However, the forces between the molecules (called intermolecular forces) are very weak.
- When a simple molecular substance melts or boils, these weak forces between molecules must be broken — not the covalent bonds within the molecules.
- Only a small amount of energy is needed to overcome these weak forces, so the substances have low melting points and boiling points.
Therefore:
\(\mathrm{Weak\ intermolecular\ forces \Rightarrow\ low\ melting\ and\ boiling\ points}\)
Examples:
- Oxygen (O₂) and chlorine (Cl₂) are gases at room temperature.
- Water (H₂O) and carbon dioxide (CO₂) have low melting and boiling points compared with ionic or giant covalent substances.
(b) Poor Electrical Conductivity
- Simple molecular substances are made up of neutral molecules.
- They do not contain ions or delocalised (free) electrons that can move through the structure.
- Because of this, they cannot carry an electric current in any state — solid, liquid, or gas.
- Therefore, simple molecular compounds are poor conductors of electricity.
\(\mathrm{No\ free\ electrons\ or\ ions \Rightarrow\ no\ electrical\ conductivity}\)
Examples:
- Hydrogen (H₂), oxygen (O₂), methane (CH₄), and carbon dioxide (CO₂) do not conduct electricity.
Example :
Explain why methane (CH₄) has a low melting point and does not conduct electricity.
▶️ Answer/Explanation
Step 1: Methane is a simple molecular substance with strong covalent bonds inside each molecule.
Step 2: The forces between the molecules are weak, so little energy is needed to separate them → low melting point.
Step 3: Methane molecules contain no free electrons or ions → cannot carry an electric current.
Final Answer: Methane has low melting and boiling points because it has weak forces between molecules and it is a poor conductor of electricity because it has no free charged particles.
