CIE iGCSE Co-ordinated Sciences-C3.2 Relative masses of atoms and molecules- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-C3.2 Relative masses of atoms and molecules – Study Notes
CIE iGCSE Co-ordinated Sciences-C3.2 Relative masses of atoms and molecules – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
- Describe relative atomic mass, Ar, as the average mass of the isotopes of an element compared to 1/12th of the mass of an atom of ¹²C
- Define relative molecular mass, Mr, as the sum of the relative atomic masses. Relative formula mass, Mr, will be used for ionic compounds
- Calculate reacting masses in simple proportions (calculations will not involve the mole concept)
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Relative Atomic Mass (\( A_r \))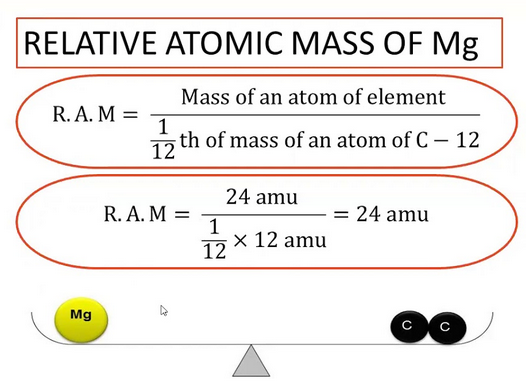
The relative atomic mass of an element is the average mass of the isotopes of that element compared to 1/12th of the mass of a carbon-12 atom.
Key points
- It takes into account the different isotopes of an element and their abundance.
- It has no units because it is a ratio.
- It is often a decimal value (not a whole number).
Formula
\( A_r = \dfrac{ \text{(Isotopic mass × % abundance)} }{100} \)
Example
Chlorine has two isotopes: \( ^{35}\text{Cl} \) (75%) and \( ^{37}\text{Cl} \) (25%). Calculate its relative atomic mass.
▶️ Answer/Explanation
\( A_r = \dfrac{(35 \times 75) + (37 \times 25)}{100} \)
\( A_r = \dfrac{2625 + 925}{100} = \dfrac{3550}{100} = 35.5 \)
Therefore, the relative atomic mass of chlorine is 35.5.
Relative Molecular Mass and Relative Formula Mass (\( M_r \))
Relative molecular mass (\( M_r \)) is the sum of the relative atomic masses (\( A_r \)) of all the atoms in a molecule.
![]()
Relative formula mass (\( M_r \)) is the sum of the relative atomic masses of all the ions shown in the formula unit of an ionic compound.
![]()
Both values are calculated from the periodic table and have no units.
Examples
Water (\( \text{H}_2\text{O} \)) → \( M_r = (2 \times 1) + 16 = 18 \)
Carbon dioxide (\( \text{CO}_2 \)) → \( M_r = 12 + (2 \times 16) = 44 \)
Ammonia (\( \text{NH}_3 \)) → \( M_r = 14 + (3 \times 1) = 17 \)
Sodium chloride (\( \text{NaCl} \)) → \( M_r = 23 + 35.5 = 58.5 \)
Calcium carbonate (\( \text{CaCO}_3 \)) → \( M_r = 40 + 12 + (3 \times 16) = 100 \)
Example
Calculate the relative formula mass of magnesium hydroxide, \( \text{Mg(OH)}_2 \).
▶️ Answer/Explanation
\( M_r = 24 + (2 \times (16 + 1)) \)
\( M_r = 24 + (2 \times 17) = 24 + 34 = 58 \)
Therefore, the relative formula mass of magnesium hydroxide is 58.
Calculating Reacting Masses (without the mole concept)
In IGCSE questions, you can find unknown reacting or product masses by using the balanced equation and simple proportionality. Treat the numbers in the equation as particle ratios, convert them to relative masses using \( A_r \) or \( M_r \), then scale to the actual mass given in the question.
![]()
Method (proportion approach)
1. Write a balanced equation.
2. Under each substance you need, write its relative mass for the amount shown in the equation (use \( A_r \) for elements, \( M_r \) for compounds).
3. Form a mass ratio between the known and the required substance.
4. Scale the ratio using the mass given in the question to find the unknown mass.
Helpful mass ratios for common equations
| Balanced equation | Relative mass on LHS | Relative mass on RHS |
|---|---|---|
| \( 2\text{H}_2 + \text{O}_2 \rightarrow 2\text{H}_2\text{O} \) | \( 2\times2 = 4 \) for \( \text{H}_2 \) and \( 32 \) for \( \text{O}_2 \) | \( 2\times18 = 36 \) for \( \text{H}_2\text{O} \) |
| \( 2\text{Mg} + \text{O}_2 \rightarrow 2\text{MgO} \) | \( 2\times24 = 48 \) for Mg and \( 32 \) for \( \text{O}_2 \) | \( 2\times40 = 80 \) for \( \text{MgO} \) |
| \( \text{CaCO}_3 \rightarrow \text{CaO} + \text{CO}_2 \) | \( 40+12+3\times16 = 100 \) for \( \text{CaCO}_3 \) | \( 56 \) for \( \text{CaO} \) and \( 44 \) for \( \text{CO}_2 \) |
Example
\( 2\text{Mg} + \text{O}_2 \rightarrow 2\text{MgO} \). If \( 12\,\text{g} \) of magnesium reacts completely with excess oxygen, calculate the mass of magnesium oxide formed.
▶️ Answer/Explanation
From the equation: \( 48\,\text{g} \) Mg → \( 80\,\text{g} \) \( \text{MgO} \).
Scale factor for Mg = \( \dfrac{12}{48} = \dfrac{1}{4} \).
So \( \text{MgO} \) mass = \( \dfrac{1}{4}\times 80 = 20\,\text{g} \).
Example
\( \text{CaCO}_3 \rightarrow \text{CaO} + \text{CO}_2 \). What mass of carbon dioxide is produced from \( 25\,\text{g} \) of pure calcium carbonate?
▶️ Answer/Explanation
From the equation: \( 100\,\text{g} \) \( \text{CaCO}_3 \) → \( 44\,\text{g} \) \( \text{CO}_2 \).
Scale factor = \( \dfrac{25}{100} = 0.25 \).
\( \text{CO}_2 \) mass = \( 0.25 \times 44 = 11\,\text{g} \).
Example
\( 2\text{H}_2 + \text{O}_2 \rightarrow 2\text{H}_2\text{O} \). If \( 3\,\text{g} \) of hydrogen reacts with excess oxygen, find the mass of water produced.
▶️ Answer/Explanation
From the equation: \( 4\,\text{g} \) \( \text{H}_2 \) → \( 36\,\text{g} \) \( \text{H}_2\text{O} \).
Scale factor = \( \dfrac{3}{4} = 0.75 \).
Water mass = \( 0.75 \times 36 = 27\,\text{g} \).
