CIE iGCSE Co-ordinated Sciences-C3.3 The mole and the Avogadro constant- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-C3.3 The mole and the Avogadro constant – Study Notes
CIE iGCSE Co-ordinated Sciences-C3.3 The mole and the Avogadro constant – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
- State that concentration can be measured in g/dm³
Supplement
- State that the mole, mol, is the unit of amount of substance and that one mole contains 6.02 × 10²³ particles, e.g. atoms, ions, molecules; this number is the Avogadro constant
- Use the relationship amount of substance (mol) = mass (g) / molar mass (g/mol) to calculate:
(a) amount of substance
(b) mass
(c) molar mass
(d) relative atomic mass or relative molecular / formula mass - Use the molar gas volume, taken as 24 dm³ at room temperature and pressure, r.t.p., in calculations involving gases
- Calculate stoichiometric reacting masses, limiting reactants, volumes of gases at r.t.p., including conversion between cm³ and dm³
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Concentration of Solutions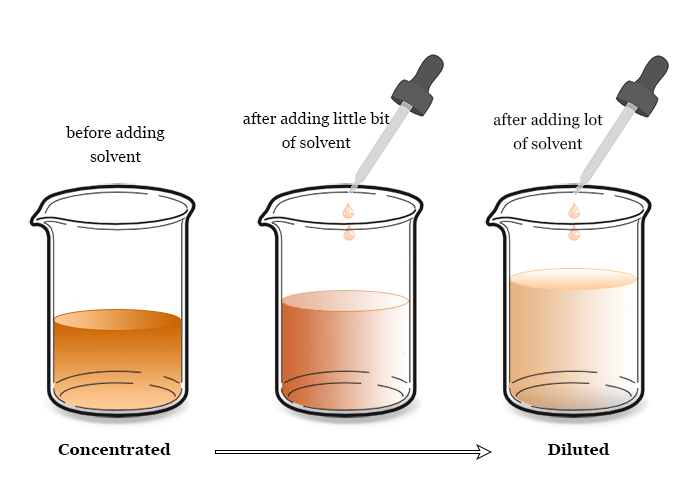
The concentration of a solution tells us how much solute is dissolved in a given volume of solvent.
Concentration can be measured in grams per cubic decimetre (\( \text{g/dm}^3 \)).
This means:
1 cubic decimetre (\( 1\,\text{dm}^3 \)) = 1 litre = 1000 cm³
So concentration in \( \text{g/dm}^3 \) = \( \dfrac{\text{mass of solute (g)}}{\text{volume of solution (dm}^3)} \)
Example
A solution contains \( 10\,\text{g} \) of sodium hydroxide dissolved in \( 250\,\text{cm}^3 \) of solution. Calculate its concentration in \( \text{g/dm}^3 \).
▶️ Answer/Explanation
First, convert volume: \( 250\,\text{cm}^3 = 0.25\,\text{dm}^3 \).
Concentration = \( \dfrac{10}{0.25} = 40\,\text{g/dm}^3 \).
The Mole and Avogadro Constant
The mole (mol) is the unit of amount of substance used in chemistry.
One mole of a substance contains \( 6.02 \times 10^{23} \) particles. These particles can be atoms, ions, or molecules depending on the substance.
This number is called the Avogadro constant.![]()
Examples
1 mole of hydrogen atoms (\( \text{H} \)) contains \( 6.02 \times 10^{23} \) atoms.
1 mole of oxygen molecules (\( \text{O}_2 \)) contains \( 6.02 \times 10^{23} \) molecules.
1 mole of sodium ions (\( \text{Na}^+ \)) contains \( 6.02 \times 10^{23} \) ions.
Example
How many molecules are present in 2 moles of carbon dioxide (\( \text{CO}_2 \))?
▶️ Answer/Explanation
1 mole of \( \text{CO}_2 \) = \( 6.02 \times 10^{23} \) molecules.
2 moles of \( \text{CO}_2 \) = \( 2 \times 6.02 \times 10^{23} \).
= \( 1.204 \times 10^{24} \) molecules.
Calculations Using the Mole Concept
The relationship between amount of substance, mass, and molar mass is:![]()
\( \text{amount of substance (mol)} = \dfrac{\text{mass (g)}}{\text{molar mass (g/mol)}} \)
This formula can be rearranged to calculate:
(a) Amount of substance: \( n = \dfrac{m}{M} \)
(b) Mass: \( m = n \times M \)
(c) Molar mass: \( M = \dfrac{m}{n} \)
(d) Relative atomic/molecular/formula mass: Use the periodic table to calculate \( M \) as the sum of relative atomic masses.
Example
Calculate the amount of substance in 18 g of water (\( \text{H}_2\text{O} \)).
▶️ Answer/Explanation
Molar mass of water: \( M = (2 \times 1) + 16 = 18\,\text{g/mol} \)
Amount of substance: \( n = \dfrac{m}{M} = \dfrac{18}{18} = 1\,\text{mol} \)
Example
Find the mass of 2 moles of carbon dioxide (\( \text{CO}_2 \)).
▶️ Answer/Explanation
Molar mass: \( M = 12 + (2 \times 16) = 44\,\text{g/mol} \)
Mass: \( m = n \times M = 2 \times 44 = 88\,\text{g} \)
Example
A sample of a compound has mass 50 g and contains 2 moles. Find its molar mass.
▶️ Answer/Explanation
Molar mass: \( M = \dfrac{m}{n} = \dfrac{50}{2} = 25\,\text{g/mol} \)
Example
Calculate the relative molecular mass of glucose (\( \text{C}_6\text{H}_{12}\text{O}_6 \)).
▶️ Answer/Explanation
\( M_r = (6 \times 12) + (12 \times 1) + (6 \times 16) = 72 + 12 + 96 = 180 \)
Molar Gas Volume at Room Temperature and Pressure (r.t.p.)
At room temperature and pressure (r.t.p., approximately 20–25°C and 1 atm), 1 mole of any gas occupies 24 dm³. The molar gas volume is the volume occupied by one mole of a gas at r.t.p.:
1 mole of gas → 24 \( \text{dm}^3 \) at r.t.p.![]()
Relationship for gas calculations
Amount of gas (mol) = \( n = \dfrac{\text{Volume of gas (dm}^3\text{)}}{24} \)
This can be rearranged to calculate:
(a) Volume of gas: \( \text{Volume (dm}^3\text{)} = n \times 24 \)
(b) Amount of substance: \( n = \dfrac{\text{Volume (dm}^3\text{)}}{24} \)
Tips
- At r.t.p., volume ratios of gases in reactions are equal to the mole ratios.
- Always use 24 \( \text{dm}^3 \) for 1 mole at r.t.p. unless specified otherwise.
- This method avoids the need to convert to grams unless the question specifically asks for mass.
Example
Calculate the amount of oxygen gas in 48 \( \text{dm}^3 \) at r.t.p.
▶️ Answer/Explanation
Amount of gas: \( n = \dfrac{48}{24} = 2\,\text{mol} \)
Example
How many \( \text{dm}^3 \) of hydrogen gas are produced from 0.5 moles at r.t.p.?
▶️ Answer/Explanation
Volume: \( \text{Volume} = n \times 24 = 0.5 \times 24 = 12\,\text{dm}^3 \)
Example
\( 2\text{H}_2 + \text{O}_2 \rightarrow 2\text{H}_2\text{O} \). If 6 \( \text{dm}^3 \) of hydrogen reacts completely with excess oxygen, find the volume of oxygen required.
▶️ Answer/Explanation
From the balanced equation, hydrogen:oxygen = 2:1 by volume.
Volume of \( \text{O}_2 \) required = \( \dfrac{1}{2} \times 6 = 3\,\text{dm}^3 \)
Stoichiometric Calculations, Limiting Reactants, and Gas Volumes at r.t.p.
These calculations use balanced chemical equations and the concept of moles. At r.t.p., 1 mole of any gas occupies 24 \( \text{dm}^3 \) or 24000 \( \text{cm}^3 \).
Key Formulas![]()
- Amount of substance (mol): \( n = \dfrac{m}{M} \)
- Volume of gas: \( V(\text{dm}^3) = n \times 24 \)
- Volume of gas: \( V(\text{cm}^3) = n \times 24000 \)
- Conversion: \( 1\,\text{dm}^3 = 1000\,\text{cm}^3 \)
Limiting Reactants
The limiting reactant is the one that gets used up first and controls the amount of product formed. Steps:
- Calculate the number of moles of each reactant
- Compare using the mole ratio from the balanced equation
- The one that gives fewer moles of product is the limiting reactant
![]()
Steps for Stoichiometric Calculations
1. Write a balanced chemical equation.
2. Calculate the moles of reactants or products using mass or volume.
3. Use the mole ratio from the balanced equation to find the moles of the unknown substance.
4. Convert moles back to mass or volume as needed.
5. Identify the limiting reactant (the one that runs out first) if more than one reactant is given.
Example :
\( 2\text{Mg} + \text{O}_2 \rightarrow 2\text{MgO} \). Calculate the mass of magnesium oxide produced from 12 g of magnesium.
▶️ Answer/Explanation
Molar masses: \( \text{Mg} = 24\,\text{g/mol} \), \( \text{MgO} = 24 + 16 = 40\,\text{g/mol} \)
Equation ratio: 2 mol Mg → 2 mol MgO = 48 g Mg → 80 g MgO
Scale factor: \( \dfrac{12}{48} = 0.25 \)
Mass of MgO produced: \( 0.25 \times 80 = 20\,\text{g} \)
Example :
\( \text{N}_2 + 3\text{H}_2 \rightarrow 2\text{NH}_3 \). If 28 g of nitrogen reacts with 6 g of hydrogen, which is the limiting reactant and how much ammonia is formed?
▶️ Answer/Explanation
Moles: \( n(\text{N}_2) = \dfrac{28}{28} = 1\,\text{mol} \), \( n(\text{H}_2) = \dfrac{6}{2} = 3\,\text{mol} \)
Mole ratio required: \( \text{N}_2:\text{H}_2 = 1:3 \) → exactly matches the moles given.
Both reactants completely react; ammonia formed: \( 2\,\text{mol} = 2 \times 17 = 34\,\text{g} \)
Example :
\( 2\text{H}_2 + \text{O}_2 \rightarrow 2\text{H}_2\text{O} \). If 6 \( \text{dm}^3 \) of hydrogen reacts completely, find the volume of oxygen required and water vapor produced.
▶️ Answer/Explanation
Mole ratio: \( \text{H}_2:\text{O}_2:\text{H}_2\text{O} = 2:1:2 \) (by volume at r.t.p.)
Volume of \( \text{O}_2 \) required: \( \dfrac{1}{2} \times 6 = 3\,\text{dm}^3 \)
Volume of \( \text{H}_2\text{O} \) produced: \( 6\,\text{dm}^3 \) (equal to H₂ reacted)
Example :
A gas occupies 5000 \( \text{cm}^3 \). Calculate its volume in \( \text{dm}^3 \) and the amount of substance at r.t.p.
▶️ Answer/Explanation
Convert to dm³: \( V = \dfrac{5000}{1000} = 5\,\text{dm}^3 \)
Amount of substance: \( n = \dfrac{V}{24} = \dfrac{5}{24} \approx 0.208\,\text{mol} \)
