CIE iGCSE Co-ordinated Sciences-C4.1 Electrolysis- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-C4.1 Electrolysis – Study Notes
CIE iGCSE Co-ordinated Sciences-C4.1 Electrolysis – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
- Define electrolysis as the decomposition of an ionic compound, when molten or in aqueous solution, by the passage of an electric current
- Identify in simple electrolytic cells:
(a) the anode as the positive electrode
(b) the cathode as the negative electrode
(c) the electrolyte as the molten or aqueous substance that undergoes electrolysis - Identify the products formed at the electrodes and describe the observations made during the electrolysis of:
(a) molten lead(II) bromide
(b) concentrated aqueous sodium chloride
(c) dilute sulfuric acid using inert electrodes made of platinum or carbon / graphite
Supplement
- Describe the transfer of charge during electrolysis:
(a) the movement of electrons in the external circuit
(b) the loss or gain of electrons at the electrodes
(c) the movement of ions in the electrolyte - Identify the products formed at the electrodes and describe the observations made during the electrolysis of aqueous copper(II) sulfate using carbon / graphite electrodes and when using copper electrodes
- State that metals or hydrogen are formed at the cathode and that non-metals (other than hydrogen) are formed at the anode
- Predict the identity of the products at each electrode for the electrolysis of a binary compound in the molten state
- Construct ionic half-equations for reactions at the cathode (showing gain of electrons as a reduction reaction)
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Electrolysis
Electrolysis is the process in which an ionic compound is decomposed by the passage of an electric current. This can occur when the compound is molten or dissolved in water (aqueous solution).
![]()
Key Points
- Molten ionic compounds: Electrolysis occurs because ions are free to move and carry charge.
- Aqueous ionic solutions: Both the ions from the dissolved compound and water can participate in the reaction.
- Electrodes: Positive electrode is the anode and attracts anions (negative ions), while the negative electrode is the cathode and attracts cations (positive ions).
- Products depend on the type of ions and whether the solution is aqueous or molten.
Example
Describe Electrolysis of molten sodium chloride (\( \text{NaCl} \))
▶️ Answer/Explanation
- Equation at cathode: \( \text{Na}^+ + e^- \rightarrow \text{Na} \)
- Equation at anode: \( 2\text{Cl}^- \rightarrow \text{Cl2} + 2e^- \)
- Overall reaction: \( 2\text{NaCl} \rightarrow 2\text{Na} + \text{Cl2} \)
Molten NaCl decomposes into sodium metal at the cathode and chlorine gas at the anode.
Electrolytic Cells:
Electrolysis is the decomposition of an ionic compound, either molten or dissolved in water, by passing an electric current through it. A simple electrolytic cell consists of three key components: electrodes, an external power source, and the electrolyte.
Key Components
Anode (positive electrode):![]()
- Attracts negative ions (anions) from the electrolyte.
- Oxidation occurs here – ions lose electrons.
- Electrons flow from the anode into the external circuit.
- Example: In molten sodium chloride, \( \text{Cl}^- \) ions are oxidized: \( 2\text{Cl}^- \rightarrow \text{Cl2} + 2e^- \).
Cathode (negative electrode):
- Attracts positive ions (cations) from the electrolyte.
- Reduction occurs here – ions gain electrons from the external circuit.
- Example: In molten sodium chloride, \( \text{Na}^+ \) ions are reduced: \( \text{Na}^+ + e^- \rightarrow \text{Na} \).
Electrolyte:
- The ionic substance that is either molten or dissolved in water.
- Contains free-moving cations and anions which carry the electric current through the cell.
- Examples: Molten \( \text{NaCl} \), aqueous \( \text{CuSO4} \) solution.
Additional Details
The flow of electrons in the external circuit is from the anode to the cathode.![]()
- The ions in the electrolyte move to the electrodes to allow the electric circuit to complete.
- Products at the electrodes depend on:
- Whether the electrolyte is molten or aqueous.
- The relative reactivity of the ions present (less reactive ions are discharged first).
Applications of electrolysis include:
- Extraction of metals like aluminium from \( \text{Al2O3} \) (Hall-Héroult process).
- Electroplating to coat objects with a layer of metal (e.g., silver plating).
- Production of chlorine and sodium hydroxide from molten or aqueous \( \text{NaCl} \).
- Purification of metals such as copper.
Example:
Describe Electrolysis of Aqueous Copper(II) Sulfate (\( \text{CuSO4} \)) using Copper Electrodes
▶️ Answer/Explanation
- Anode (positive): Copper dissolves into ions: \( \text{Cu} \rightarrow \text{Cu}^{2+} + 2e^- \)
- Cathode (negative): Copper ions are reduced and deposit on the cathode: \( \text{Cu}^{2+} + 2e^- \rightarrow \text{Cu} \)
- Electrolyte: \( \text{CuSO4} \) provides \( \text{Cu}^{2+} \) and \( \text{SO4}^{2-} \) ions to carry current.
Overall effect: Copper is transferred from the anode to the cathode, keeping the solution composition almost unchanged.
Electrolysis of Different Substances with Inert Electrodes
When inert electrodes (platinum or carbon/graphite) are used, the products of electrolysis depend on whether the substance is molten or aqueous and the ions present.
(a) Molten Lead(II) Bromide (\( \rm{PbBr_2} \))
![]()
- Cathode (negative electrode): Lead ions (\( \text{Pb}^{2+} \)) are reduced to form molten lead: \( \text{Pb}^{2+} + 2e^- \rightarrow \text{Pb} \)
- Anode (positive electrode): Bromide ions (\( \text{Br}^- \)) are oxidized to form bromine gas: \( 2\text{Br}^- \rightarrow \text{Br2} + 2e^- \)
- Observations: Silvery molten lead collects at the cathode; reddish-brown bromine gas is seen at the anode.
(b) Concentrated Aqueous Sodium Chloride (\( \rm{NaCl} \))
![]()
- Cathode (negative electrode): Hydrogen ions (\( \text{H}^+ \)) from water are reduced: \( 2\text{H}^+ + 2e^- \rightarrow \text{H2} \)
- Anode (positive electrode): Chloride ions (\( \text{Cl}^- \)) are oxidized: \( 2\text{Cl}^- \rightarrow \text{Cl2} + 2e^- \)
- Observations: Bubbles of hydrogen gas at the cathode; yellow-green chlorine gas at the anode.
(c) Dilute Sulfuric Acid (\( \rm{H_2SO_4} \))
![]()
- Cathode (negative electrode): Hydrogen ions (\( \text{H}^+ \)) are reduced: \( 2\text{H}^+ + 2e^- \rightarrow \text{H2} \)
- Anode (positive electrode): Hydroxide ions (\( \text{OH}^- \)) from water are oxidized: \( 4\text{OH}^- \rightarrow O2 + 2\text{H2O} + 4e^- \)
- Observations: Bubbles of hydrogen gas at the cathode; bubbles of oxygen gas at the anode.
Example
An electrolytic cell is set up with concentrated aqueous sodium chloride (\( \text{NaCl} \)) using inert platinum electrodes. Predict the products formed at the electrodes and describe the observations. Also, write the half-equations and the overall reaction.
▶️ Answer/Explanation
Step 1: Identify ions in solution
- Cations: \( \text{Na}^+ \), \( \text{H}^+ \) (from water)
- Anions: \( \text{Cl}^- \), \( \text{OH}^- \) (from water)
Step 2: Determine products at each electrode
- Cathode (negative): Hydrogen ions are less reactive than sodium, so hydrogen gas is produced: \( 2\text{H}^+ + 2e^- \rightarrow \text{H2} \)
- Anode (positive): Chloride ions are discharged to form chlorine gas: \( 2\text{Cl}^- \rightarrow \text{Cl2} + 2e^- \)
Step 3: Overall reaction
\( 2\text{H}^+ + 2\text{Cl}^- \rightarrow \text{H2} + \text{Cl2} \)
Step 4: Observations
- Bubbles of hydrogen gas form at the cathode.
- Yellow-green chlorine gas forms at the anode.
Step 5: Notes
- Platinum electrodes are inert and do not react themselves.
- The electrolyte conducts electricity because of the free-moving ions.
Transfer of Charge During Electrolysis
Electrolysis involves the movement of charge through the external circuit and within the electrolyte. This ensures that oxidation and reduction reactions occur continuously at the electrodes.
(a) Movement of electrons in the external circuit: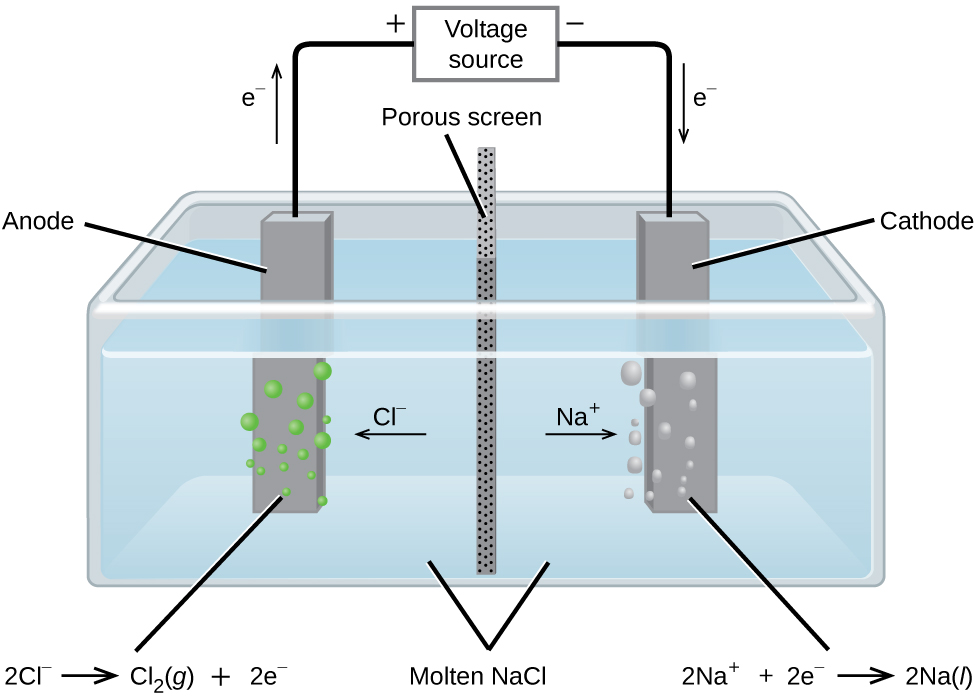
- Electrons flow from the negative terminal of the power supply to the cathode (negative electrode).
- Electrons leave the anode (positive electrode) and flow back to the positive terminal of the power supply.
- This flow of electrons allows reduction at the cathode and oxidation at the anode.
(b) Loss or gain of electrons at the electrodes:
- At the cathode, cations gain electrons (reduction)
- At the anode, anions lose electrons (oxidation)
- This electron transfer at electrodes drives the chemical reactions of electrolysis.
(c) Movement of ions in the electrolyte:
- Cations (positive ions) move toward the cathode to gain electrons.
- Anions (negative ions) move toward the anode to lose electrons.
- This movement completes the internal circuit and allows continuous current flow through the electrolyte.
Example
Aqueous copper(II) sulfate (\( \text{CuSO4} \)) is electrolysed using inert carbon electrodes. Explain the transfer of charge during this process and identify the products formed at each electrode.
▶️ Answer/Explanation
Step 1: Electrons in the external circuit
- Electrons flow from the negative terminal of the power supply to the cathode and from the anode back to the positive terminal.
Step 2: Electron transfer at electrodes
- Cathode: Copper ions are reduced: \( \text{Cu}^{2+} + 2e^- \rightarrow \text{Cu} \)
- Anode: Oxidation of water produces oxygen: \( 2\text{H2O} \rightarrow O2 + 4\text{H}^+ + 4e^- \)
Step 3: Movement of ions in the electrolyte
- Cations (\( \text{Cu}^{2+} \)) move toward the cathode.
- Anions (\( \text{SO4}^{2-} \)) and water molecules balance the charge and allow current to flow toward the anode.
Observations: Reddish-brown copper deposits on the cathode; bubbles of oxygen at the anode; solution remains blue due to dissolved \( \text{Cu}^{2+} \) ions.
Electrolysis of Aqueous Copper(II) Sulfate
The products formed during the electrolysis of aqueous \( \text{CuSO4} \) depend on the type of electrodes used: inert (carbon/graphite) or reactive (copper).
(a) Using Carbon/Graphite Electrodes (Inert)
![]()
- Cathode (negative electrode): Copper ions (\( \text{Cu}^{2+} \)) are reduced to copper metal: \( \text{Cu}^{2+} + 2e^- \rightarrow \text{Cu} \)
- Anode (positive electrode): Water may be slightly oxidized, but mainly oxygen is released if sulfate ions are not discharged: \( 2\text{H2O} \rightarrow O2 + 4\text{H}^+ + 4e^- \)
- Observations: Reddish-brown copper deposits form on the cathode; bubbles of oxygen may appear at the anode.
(b) Using Copper Electrodes (Reactive)
![]()
- Cathode (negative electrode): Copper ions from the solution are reduced and deposit on the cathode: \( \text{Cu}^{2+} + 2e^- \rightarrow \text{Cu} \)
- Anode (positive electrode): Copper metal from the anode dissolves to form \( \text{Cu}^{2+} \) ions: \( \text{Cu} \rightarrow \text{Cu}^{2+} + 2e^- \)
- Observations: The cathode gains a layer of copper; the anode gradually decreases in mass as copper dissolves; the solution remains blue due to constant \( \text{Cu}^{2+} \) concentration.
Example
Aqueous copper(II) sulfate is electrolysed using:
- (i) Carbon electrodes
- (ii) Copper electrodes
Predict the products formed at each electrode and describe the observations in each case.
▶️ Answer/Explanation
(i) Carbon electrodes:
Cathode: Copper metal deposited (\( \text{Cu}^{2+} + 2e^- \rightarrow \text{Cu} \))
Anode: Oxygen gas may evolve from water (\( 2\text{H2O} \rightarrow O2 + 4\text{H}^+ + 4e^- \))
Observation: Reddish-brown copper on cathode; bubbles of oxygen at anode.
(ii) Copper electrodes:
Cathode: Copper ions reduced and deposit on cathode (\( \text{Cu}^{2+} + 2e^- \rightarrow \text{Cu} \))
Anode: Copper dissolves into solution (\( \text{Cu} \rightarrow \text{Cu}^{2+} + 2e^- \))
Observation: Cathode gains copper; anode loses mass; solution stays blue.
Products Formed at Electrodes
During electrolysis, the type of product formed depends on the charge of the ions and the reactivity of the elements involved.
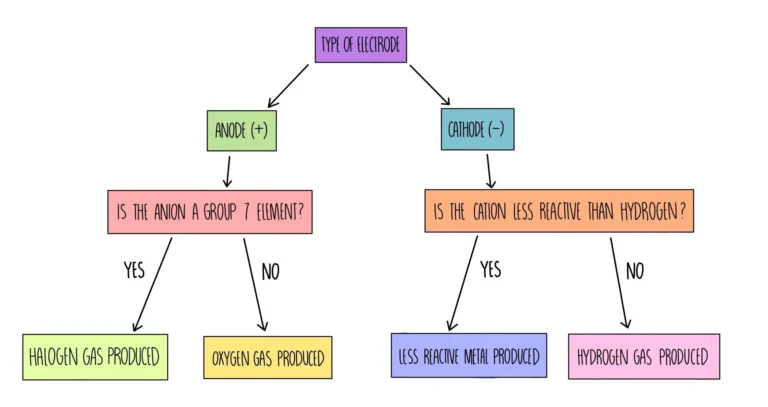
Cathode (negative electrode):
Attracts positive ions (cations).
Metals or hydrogen are formed here, depending on the ion present:
- If a metal is less reactive than hydrogen, the metal is deposited.
- If the cation is from a reactive metal (like Na⁺, K⁺) in aqueous solution, hydrogen gas is produced instead.
Anode (positive electrode):
Attracts negative ions (anions).
Non-metals (other than hydrogen) are formed here. For example:
- Halide ions (Cl⁻, Br⁻, I⁻) are discharged to form halogen gases $\rm{(Cl_2, Br_2, I_2)}$.
- If no halide ions are present, oxygen is produced from water oxidation.
Example
During the electrolysis of aqueous sodium chloride using inert electrodes, identify the products formed at the cathode and anode and explain why.
▶️ Answer/Explanation
- Cathode: Hydrogen gas is formed because sodium is more reactive than hydrogen: \( 2\text{H}^+ + 2e^- \rightarrow \text{H2} \)
- Anode: Chlorine gas is formed because Cl⁻ ions are discharged preferentially over OH⁻: \( 2\text{Cl}^- \rightarrow \text{Cl2} + 2e^- \)
- Observation: Bubbles of hydrogen at the cathode; yellow-green chlorine gas at the anode.
Electrolysis of Molten Binary Compounds
Binary compounds consist of two elements, usually a metal and a non-metal. When molten, they conduct electricity because ions are free to move. The products at the electrodes can be predicted using the following rules:
Cathode (negative electrode):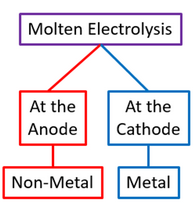
- Attracts positive ions (cations).
- The metal is formed at the cathode by reduction: \( \text{M}^{n+} + ne^- \rightarrow \text{M} \)
Anode (positive electrode):
- Attracts negative ions (anions).
- The non-metal is formed at the anode by oxidation: \( 2\text{X}^- \rightarrow \text{X2} + 2e^- \)
Example
Predict the products formed at the electrodes when molten lead(II) bromide (\( \text{PbBr2} \)) is electrolysed using inert carbon electrodes. Describe the observations.
▶️ Answer/Explanation
- Cathode (negative electrode): Lead ions are reduced to molten lead: \( \text{Pb}^{2+} + 2e^- \rightarrow \text{Pb} \)
- Anode (positive electrode): Bromide ions are oxidized to bromine gas: \( 2\text{Br}^- \rightarrow \text{Br2} + 2e^- \)
- Observations: Silvery molten lead collects at the cathode; reddish-brown bromine gas bubbles form at the anode.
Cathode Reactions: Ionic Half-Equations
At the cathode (negative electrode), cations gain electrons in a reduction reaction. The general rule is: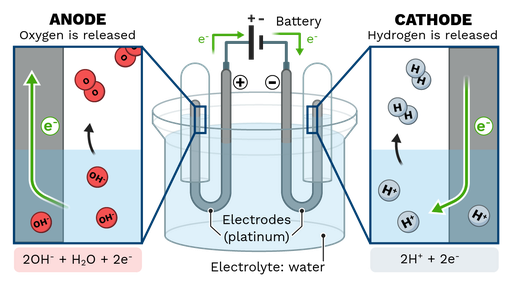
Cation + electrons → Element
Example : Hydrogen ions from dilute acid
- Cation: \( \text{H}^+ \)
- Half-equation: \( 2\text{H}^+ + 2e^- \rightarrow \text{H2} \)
- Observation: Bubbles of hydrogen gas at the cathode.
Example : Sodium ions in molten sodium chloride
- Cation: \( \text{Na}^+ \)
- Half-equation: \( \text{Na}^+ + e^- \rightarrow \text{Na} \)
- Observation: Molten sodium forms at the cathode.
Key Points:
- The cathode is always negative, attracting cations.
- Reduction always involves gain of electrons.
- The product is either a metal or hydrogen gas, depending on the cation.
Example
Write the ionic half-equation for the reduction of copper(II) ions at the cathode during the electrolysis of aqueous copper(II) sulfate using inert electrodes. Describe the observation.
▶️ Answer/Explanation
- Half-equation: \( \text{Cu}^{2+} + 2e^- \rightarrow \text{Cu} \)
- Observation: Reddish-brown copper deposits on the cathode.
