CIE iGCSE Co-ordinated Sciences-C4.2 Hydrogen–oxygen fuel cells- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-C4.2 Hydrogen–oxygen fuel cells – Study Notes
CIE iGCSE Co-ordinated Sciences-C4.2 Hydrogen–oxygen fuel cells – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
- State that a hydrogen–oxygen fuel cell uses hydrogen and oxygen to produce electricity with water as the only chemical product
Supplement
- Describe the advantages and disadvantages of using hydrogen–oxygen fuel cells in comparison with gasoline / petrol engines in vehicles
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
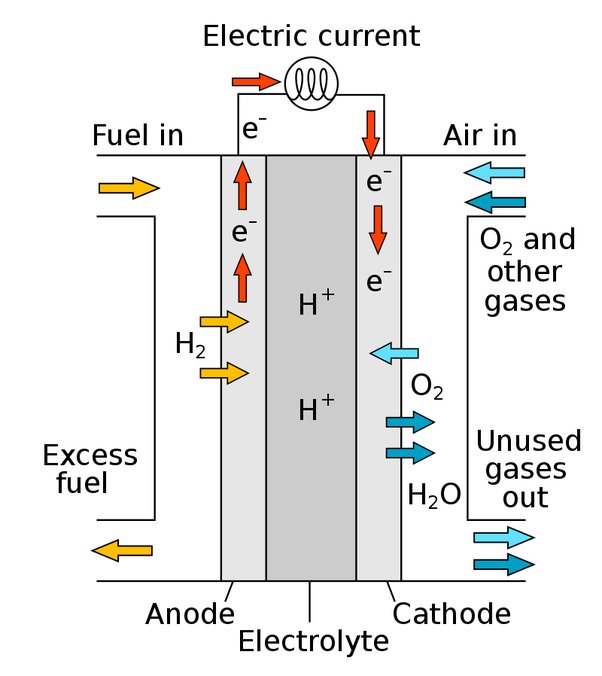
Hydrogen–Oxygen Fuel Cell
A hydrogen–oxygen fuel cell is a device that converts the chemical energy of hydrogen and oxygen directly into electrical energy. The only chemical product of the reaction is water, making it a clean energy source.
How it works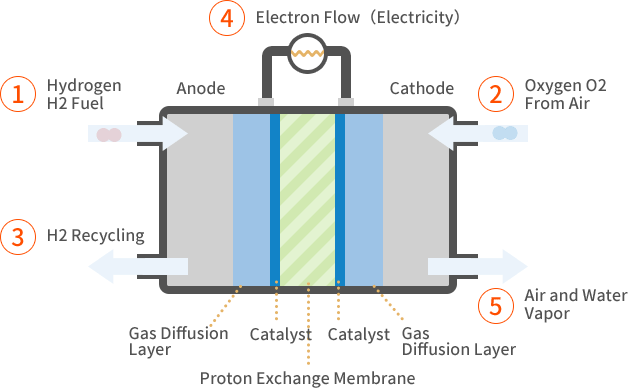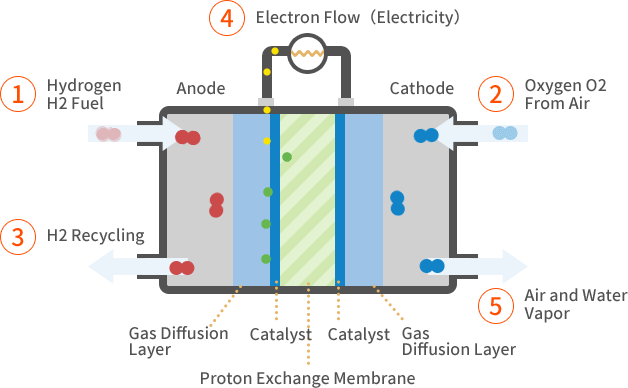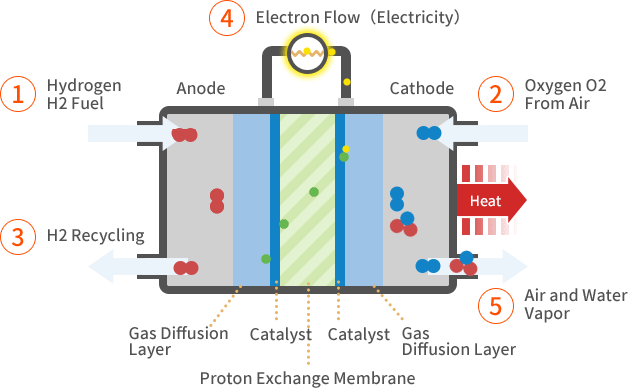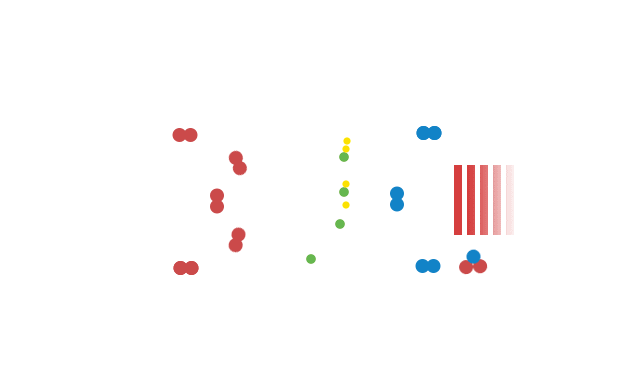
- Hydrogen gas (\( \text{H}_2 \)) is supplied to the anode (negative electrode).
- Oxygen gas (\( \text{O}_2 \)) is supplied to the cathode (positive electrode).
- At the anode, hydrogen molecules are oxidized, releasing electrons and forming hydrogen ions (protons):
\( \text{H}_2 \rightarrow 2\text{H}^+ + 2e^- \) - The electrons flow through an external circuit, producing electric current.
- At the cathode, oxygen molecules react with the electrons and hydrogen ions to form water:
\( \dfrac{1}{2}\text{O}_2 + 2\text{H}^+ + 2e^- \rightarrow \text{H}_2\text{O} \) - Overall reaction in the fuel cell:
\( 2\text{H}_2 + \text{O}_2 \rightarrow 2\text{H}_2\text{O} \)
 Advantages
Advantages
- Produces electricity efficiently with minimal energy loss.
- The only chemical product is water, so it does not produce harmful emissions.
- Can operate continuously as long as hydrogen and oxygen are supplied.
Applications
- Fuel cells are used in hydrogen-powered vehicles, such as cars and buses.
- They provide backup power for buildings and remote installations.
- They are used in spacecraft for both electricity and water generation.
Example
In a hydrogen–oxygen fuel cell, 4 moles of hydrogen react with oxygen. Calculate:
- The moles of water produced.
- The volume of water vapor produced at r.t.p. (1 mole of gas = 24 \( \text{dm}^3 \)).
▶️ Answer/Explanation
Balanced equation: \( 2\text{H}_2 + \text{O}_2 \rightarrow 2\text{H}_2\text{O} \)
- Mole ratio H₂ : H₂O = 2 : 2 = 1 : 1
- Moles of H₂ given = 4 → moles of H₂O produced = 4
- Volume of water vapor at r.t.p.: \( V = n \times 24 = 4 \times 24 = 96\,\text{dm}^3 \)
Example
A hydrogen–oxygen fuel cell is used to power a vehicle. 10 moles of hydrogen react completely with oxygen. Calculate:
- The moles of water produced.
- The volume of water vapor produced at r.t.p. (1 mole of gas = 24 \( \text{dm3} \)).
- The total number of water molecules produced.
▶️ Answer/Explanation
Step 1: Write the balanced chemical equation
\( 2\text{H2} + \text{O2} \rightarrow 2\text{H2O} \)
Step 2: Determine the mole ratio
- Mole ratio H2 : H2O = 2 : 2 = 1 : 1
- Moles of H2 given = 10 → moles of H2O produced = 10
Step 3: Calculate the volume of water vapor at r.t.p.
- \( V = n \times 24 = 10 \times 24 = 240\,\text{dm3} \)
Step 4: Calculate the total number of water molecules
- Number of molecules = moles × Avogadro constant
- \( N = 10 \times 6.02 \times 10^{23} \approx 6.02 \times 10^{24} \) molecules
Advantages and Disadvantages of Hydrogen–Oxygen Fuel Cells Compared to Gasoline Engines
Advantages
- Produces electricity efficiently with minimal energy loss because chemical energy is directly converted into electrical energy without intermediate combustion.
- The only chemical product is water (\( \text{H2O} \)), so it does not emit carbon dioxide (\( \text{CO2} \)) or other greenhouse gases, reducing global warming potential.
- Reduces air pollution in urban areas compared to petrol engines that produce carbon monoxide (\( \text{CO} \)), nitrogen oxides (\( \text{NOx} \)), and particulate matter.
- Can operate continuously as long as hydrogen (\( \text{H2} \)) and oxygen (\( \text{O2} \)) are supplied, making it suitable for long-duration use.
- Quiet operation due to no combustion, leading to lower noise pollution.
- Refueling with hydrogen can be faster than charging electric batteries, making it convenient for certain applications like buses and trucks.
Disadvantages
- Hydrogen storage and transport are challenging because hydrogen (\( \text{H2} \)) is highly flammable and requires high-pressure tanks or cryogenic storage.
- Fuel cell vehicles currently have higher initial costs compared to conventional petrol vehicles due to expensive catalysts like platinum.
- Hydrogen production is often energy-intensive and may rely on fossil fuels, which can reduce the overall environmental benefit if not produced sustainably.
- Limited refueling infrastructure for hydrogen (\( \text{H2} \)) compared to widespread petrol stations, restricting travel range and convenience.
- Fuel cells can degrade over time, and replacement costs can be high.
Example
A hydrogen–oxygen fuel cell car uses 4 moles of hydrogen. Compare the energy output and environmental impact with a petrol car that burns an equivalent amount of fuel. Calculate the mass of water produced and the total number of water molecules.
▶️ Answer/Explanation
Step 1: Balanced chemical equation
\( 2\text{H2} + \text{O2} \rightarrow 2\text{H2O} \)
Step 2: Mole ratio and water production
- Moles of H2 = 4 → moles of H2O produced = 4 (1:1 ratio)
- Molar mass of H2O = 18 g/mol → mass of water = 4 × 18 = 72 g
Step 3: Number of water molecules
- Number of molecules = moles × Avogadro constant
- \( N = 4 \times 6.02 \times 10^{23} \approx 2.41 \times 10^{24} \) molecules
Step 4: Environmental comparison
- Fuel cell produces only water, no CO2 or NOx emissions.
- Petrol combustion releases CO2, CO, and NOx, contributing to air pollution and global warming.
- Hydrogen fuel cell car is more environmentally friendly and efficient.
