CIE iGCSE Co-ordinated Sciences-C5.1 Exothermic and endothermic reactions- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-C5.1 Exothermic and endothermic reactions – Study Notes
CIE iGCSE Co-ordinated Sciences-C5.1 Exothermic and endothermic reactions – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
- State that an exothermic reaction transfers thermal energy to the surroundings leading to an increase in the temperature of the surroundings
- State that an endothermic reaction takes in thermal energy from the surroundings leading to a decrease in the temperature of the surroundings
Supplement
- Interpret reaction pathway diagrams showing exothermic and endothermic reactions
- State that the transfer of thermal energy during a reaction is called the enthalpy change, ∆H, of the reaction. ∆H is negative for exothermic reactions and positive for endothermic reactions
- Define activation energy, Ea, as the minimum energy that colliding particles must have to react
- Draw and label reaction pathway diagrams for exothermic and endothermic reactions using information provided, to include:
(a) reactants
(b) products
(c) overall energy change of the reaction, ∆H
(d) activation energy, Ea - State that bond breaking is an endothermic process and bond making is an exothermic process
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Exothermic Reactions
An exothermic reaction is a chemical reaction that transfers thermal energy to the surroundings. This results in an increase in the temperature of the surroundings.
Explanation:![]()
- During an exothermic reaction, energy is released when new bonds are formed in the products.
- The amount of energy released from bond formation is greater than the energy absorbed to break bonds in the reactants.
- This extra energy is given out as heat (thermal energy) to the surroundings.
- The surroundings become hotter and the temperature rises.
\(\mathrm{Chemical \ energy → Thermal \ energy}\)
Examples of Exothermic Reactions:
- Combustion: e.g. \(\mathrm{CH_4 + 2O_2 → CO_2 + 2H_2O}\)
- Neutralisation: e.g. \(\mathrm{HCl + NaOH → NaCl + H_2O}\)
- Respiration: \(\mathrm{C_6H_{12}O_6 + 6O_2 → 6CO_2 + 6H_2O}\)
- Oxidation of metals: e.g. \(\mathrm{2Mg + O_2 → 2MgO}\)
Everyday Examples:
- Burning fuels (candles, gas, wood).
- Hand warmers releasing heat.
- Combustion in engines.
Example :
State what happens to the temperature of the surroundings during an exothermic reaction and explain why.
▶️ Answer/Explanation
Step 1: In an exothermic reaction, energy is released to the surroundings.
Step 2: This energy transfer increases the thermal energy of the surroundings.
Final Answer: The temperature of the surroundings increases because the reaction transfers heat energy to the surroundings.
Endothermic Reactions
An endothermic reaction is a chemical reaction that takes in thermal energy from the surroundings. This results in a decrease in the temperature of the surroundings.
Explanation:![]()
- During an endothermic reaction, energy is absorbed to break bonds in the reactants.
- The amount of energy absorbed to break bonds is greater than the energy released when new bonds are formed.
- The extra energy needed is taken from the surroundings as heat.
- This causes the surroundings to become colder — the temperature decreases.
\(\mathrm{Thermal \ energy \ from \ surroundings → Chemical \ energy}\)
Examples of Endothermic Reactions:
- Thermal decomposition: \(\mathrm{CaCO_3 → CaO + CO_2}\)
- Photosynthesis: \(\mathrm{6CO_2 + 6H_2O → C_6H_{12}O_6 + 6O_2}\)
- Dissolving ammonium salts: \(\mathrm{NH_4Cl(s) → NH_4^+ (aq) + Cl^- (aq)}\)
- Electrolysis: requires energy input to drive the reaction.
Everyday Examples:
- Instant cold packs used for injuries (absorb heat from surroundings).
- Photosynthesis in plants — absorbs light and heat energy.
Example :
State what happens to the temperature of the surroundings during an endothermic reaction and explain why.
▶️ Answer/Explanation
Step 1: In an endothermic reaction, energy is taken in from the surroundings.
Step 2: This energy is used to break bonds, so less heat remains in the surroundings.
Final Answer: The temperature of the surroundings decreases because the reaction absorbs thermal energy from them.
Reaction Pathway Diagrams: Exothermic and Endothermic Reactions
Reaction pathway diagrams (also called energy profile diagrams) show the progress of a chemical reaction and the energy changes that occur from reactants to products.
Exothermic reactions: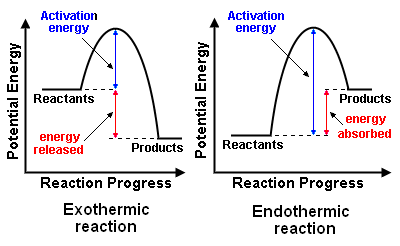
- The energy of the products is lower than the energy of the reactants.
- Energy is released to the surroundings, often as heat or light.
- The diagram shows a downward slope from reactants to products.
- Activation energy is the energy peak that must be overcome for the reaction to proceed.
Endothermic reactions:
- The energy of the products is higher than the energy of the reactants.
- Energy is absorbed from the surroundings to allow the reaction to occur.
- The diagram shows an upward slope from reactants to products.
- Activation energy is still required to initiate the reaction.
Example
Explain how you can identify whether a reaction is exothermic or endothermic by looking at its reaction pathway diagram.
▶️ Answer/Explanation
- Compare the energy levels of reactants and products.
- If the products are lower in energy than the reactants, the reaction is exothermic; energy is released to the surroundings.
- If the products are higher in energy than the reactants, the reaction is endothermic; energy is absorbed from the surroundings.
- The activation energy is the peak of the curve, representing the minimum energy required to start the reaction.
Enthalpy Change (\( \Delta H \))
![]()
The transfer of thermal energy during a chemical reaction is called the enthalpy change, \( \Delta H \), of the reaction. It represents the difference in energy between reactants and products. Understanding enthalpy change helps predict whether a reaction will release or absorb energy, and it is crucial in practical applications such as fuels, batteries, and industrial processes.
Exothermic reactions:![]()
- Energy is released to the surroundings, usually as heat or light.
- \( \Delta H \) is negative (\( \Delta H < 0 \)).
- The products have lower energy than the reactants.
- Common examples: combustion of fuels, neutralisation reactions, respiration.
- Observation: Temperature of the surroundings increases.
- Applications: Heating systems, hand warmers, combustion engines.
Endothermic reactions:
- Energy is absorbed from the surroundings, usually as heat.

- \( \Delta H \) is positive (\( \Delta H > 0 \)).
- The products have higher energy than the reactants.
- Common examples: thermal decomposition, photosynthesis, dissolving ammonium chloride in water.
- Observation: Temperature of the surroundings decreases.
- Applications: Instant cold packs, photosynthesis in plants, certain industrial processes.
Additional Points:
- Enthalpy change depends on the type and number of bonds broken and formed.
- Energy profile diagrams can show the relative energy levels of reactants and products and the activation energy.
- Measuring \( \Delta H \) helps chemists design reactions efficiently and safely.
Example
A reaction releases 250 kJ of energy to the surroundings. State the sign of \( \Delta H \), identify whether the reaction is exothermic or endothermic, and explain what happens to the temperature of the surroundings.
▶️ Answer/Explanation
- Energy is released → heat flows to the surroundings.
- \( \Delta H \) is negative: \( \Delta H = -250\,\text{kJ} \).
- The reaction is exothermic.
- The temperature of the surroundings increases due to the released energy.
Activation Energy (\( E_a \))
Activation energy, \( E_a \), is the minimum energy that colliding particles must have for a chemical reaction to occur. Without this energy, even if particles collide, the reaction will not proceed. It is an essential concept in understanding reaction rates and energy profile diagrams.
![]()
- Represents the energy barrier between reactants and products.
- Determines how quickly a reaction occurs; higher \( E_a \) → slower reaction at a given temperature.
- Can be lowered by a catalyst, which provides an alternative pathway with a lower activation energy.
- Shown as the peak of a curve in a reaction pathway or energy profile diagram.
Example
In a reaction, the reactants have an energy of 50 kJ/mol, and the peak of the reaction pathway diagram is at 120 kJ/mol. Calculate the activation energy and explain its significance.
▶️ Answer/Explanation
- Activation energy: \( E_a = 120 – 50 = 70\,\text{kJ/mol} \)
- This is the minimum energy the particles must have to overcome the energy barrier and react.
- Particles with energy less than \( E_a \) will collide but will not form products.
Reaction Pathway Diagrams:
(a) Reactants![]()
In a reaction pathway diagram, the reactants are the substances present at the start of a chemical reaction. They are shown on the left-hand side of the diagram and their position on the y-axis represents their relative energy.
- For both exothermic and endothermic reactions, reactants appear at the beginning of the energy curve.
- Their energy level serves as a reference point to compare with the products.
(b) Products
In a reaction pathway diagram, the products are the substances formed at the end of the reaction. They are shown on the right-hand side of the diagram, and their position on the y-axis represents their relative energy compared to the reactants.
- For an exothermic reaction, the products have lower energy than the reactants because energy is released to the surroundings.
- For an endothermic reaction, the products have higher energy than the reactants because energy is absorbed from the surroundings.
(c) Overall Energy Change (\( \Delta H \))
The overall energy change of a reaction, \( \Delta H \), is the difference in energy between the reactants and the products. It indicates whether a reaction is exothermic or endothermic.
![]()
- For an exothermic reaction, \( \Delta H \) is negative (\( \Delta H < 0 \)) because the products have lower energy than the reactants. Energy is released to the surroundings.
- For an endothermic reaction, \( \Delta H \) is positive (\( \Delta H > 0 \)) because the products have higher energy than the reactants. Energy is absorbed from the surroundings.
- The magnitude of \( \Delta H \) is represented by the vertical difference between reactants and products on the energy axis of the reaction pathway diagram.
(d) Activation Energy (\( E_a \))
Activation energy, \( E_a \), is the minimum energy that reacting particles must have for a reaction to occur. On a reaction pathway diagram, it is represented as the energy difference between the reactants and the peak of the curve (the transition state).
![]()
- For both exothermic and endothermic reactions, the curve rises from the reactants to a maximum point representing \( E_a \).
- After reaching the peak, the curve falls to the products’ energy level.
- The larger the activation energy, the slower the reaction occurs at a given temperature.
- Catalysts work by providing an alternative pathway with a lower \( E_a \), increasing the reaction rate.
Example
On a reaction pathway diagram for the combustion of methane, label the reactants. The reaction is: \( \text{CH}_{4} + 2\text{O}_{2} \;\rightarrow\; \text{CO}_{2} + 2\text{H}_{2}\text{O} \).
▶️ Answer/Explanation
- Reactants: \( \text{CH}_{4} \) and \( \text{O}_{2} \)
- They are positioned at the start of the energy curve (left-hand side).
- This shows the initial energy of the system before the reaction begins.
Example
On a reaction pathway diagram for the combustion of methane, label the products. The reaction is: \( \text{CH}_{4} + 2\text{O}_{2} \;\rightarrow\; \text{CO}_{2} + 2\text{H}_{2}\text{O} \).
▶️ Answer/Explanation
- Products: \( \text{CO}_{2} \) and \( \text{H}_{2}\text{O} \)
- They are positioned at the end of the energy curve (right-hand side).
- For this exothermic reaction, the products have lower energy than the reactants, indicating energy is released to the surroundings
Example
Using a reaction pathway diagram for the combustion of methane (\( \text{CH}_4 + 2\text{O}_2 \rightarrow \text{CO}_2 + 2\text{H}_2\text{O} \)), determine the overall energy change (\( \Delta H \)) and explain its sign.
▶️ Answer/Explanation
- Reactants: \( \text{CH}_4 \) and \( \text{O}_2 \); Products: \( \text{CO}_2 \) and \( \text{H}_2\text{O} \)
- Products have lower energy than reactants → energy released.
- Overall energy change: \( \Delta H < 0 \), indicating an exothermic reaction.
- The vertical difference between reactants and products on the diagram shows the magnitude of \( \Delta H \).
Example
For the combustion of methane (\( \text{CH}_4 + 2\text{O}_2 \rightarrow \text{CO}_2 + 2\text{H}_2\text{O} \)), the reactants have an energy of 50 kJ/mol, and the peak of the reaction pathway is at 120 kJ/mol. Calculate the activation energy (\( E_a \)) and explain its significance.
▶️ Answer/Explanation
- Activation energy: \( E_a = 120 – 50 = 70\,\text{kJ/mol} \)
- This is the minimum energy required for methane and oxygen molecules to collide successfully and react.
- Particles with energy less than \( E_a \) will collide but will not form products.
Bond Energy: Breaking and Making Bonds
In chemical reactions, energy is involved in breaking and forming bonds. This is an important concept for understanding why reactions are exothermic or endothermic.![]()
Bond breaking: Energy is required to break chemical bonds between atoms. Therefore, bond breaking is an endothermic process and absorbs energy from the surroundings.
Bond making: Energy is released when new chemical bonds are formed. Therefore, bond making is an exothermic process and releases energy to the surroundings.
The overall energy change of a reaction (\( \Delta H \)) depends on the difference between the energy absorbed to break bonds and the energy released when new bonds are formed:
- If more energy is released in bond formation than absorbed in bond breaking → reaction is exothermic (\( \Delta H < 0 \)).
- If more energy is absorbed in bond breaking than released in bond formation → reaction is endothermic (\( \Delta H > 0 \)).
Example
During the reaction \( \text{H}_2 + \text{Cl}_2 \rightarrow 2\text{HCl} \), explain why bond breaking and bond making affect the overall energy change.
▶️ Answer/Explanation
- Energy is required to break the H-H and Cl-Cl bonds → endothermic process.
- Energy is released when two H-Cl bonds are formed → exothermic process.
- If the energy released in forming H-Cl bonds is greater than the energy absorbed in breaking H-H and Cl-Cl bonds, the overall reaction is exothermic.
