CIE iGCSE Co-ordinated Sciences-C6.2 Rate of reaction- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-C6.2 Rate of reaction – Study Notes
CIE iGCSE Co-ordinated Sciences-C6.2 Rate of reaction – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
- Describe the effect on the rate of reaction of:
(a) changing the concentration of solutions
(b) changing the pressure of gases
(c) changing the surface area of solids
(d) changing the temperature
(e) adding or removing a catalyst - State that a catalyst increases the rate of a reaction and is unchanged at the end of a reaction
- Describe practical methods for investigating the rate of a reaction including change in mass of a reactant or product and the formation of a gas
- Interpret data, including graphs, from rate of reaction experiments
Supplement
- Explain the effect on the rate of reaction of:
(a) changing the concentration of solutions
(b) changing the pressure of gases
(c) changing the surface area of solids
(d) changing the temperature
(e) adding or removing a catalyst using collision theory - State that a catalyst decreases the activation energy, Ea, of a reaction
- Describe collision theory in terms of:
(a) number of particles per unit volume
(b) frequency of collisions between particles
(c) kinetic energy of particles
(d) activation energy, Ea
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Effect of Changing the Concentration of Solutions on Reaction Rate
The concentration of a solution is the amount of solute (reactant) present in a given volume of solvent. Changing the concentration directly affects the rate of a chemical reaction involving aqueous solutions.
How concentration affects reaction rate:![]()
- Increasing the concentration increases the number of reactant particles per unit volume.
- More particles in the same volume lead to more frequent collisions between reactant particles.
- Frequent collisions increase the probability that particles will collide with sufficient energy to react (greater collision frequency).
- As a result, the reaction rate increases with higher concentration.
Conversely:
- Decreasing the concentration reduces the number of reactant particles per unit volume.
- Fewer collisions occur, leading to a slower reaction.
Examples:
- Hydrochloric acid reacting with magnesium: A more concentrated HCl solution produces hydrogen gas faster than a dilute solution.
- Sodium thiosulfate reacting with hydrochloric acid: Increasing the concentration of either reactant makes the sulfur precipitate form more quickly.
Example
Predict what happens to the rate of reaction when the concentration of hydrochloric acid is doubled while reacting with magnesium ribbon.
▶️ Answer/Explanation
- Doubling the HCl concentration increases the number of HCl particles per unit volume.
- This leads to more frequent collisions with magnesium atoms.
- Reaction rate increases → hydrogen gas is produced faster.
Effect of Changing the Pressure of Gases on Reaction Rate
Pressure affects the rate of reaction only for reactions involving gaseous reactants. Changing the pressure effectively changes the concentration of gas particles in a given volume.
How pressure affects reaction rate:![]()
- Increasing the pressure compresses gas molecules into a smaller volume, increasing the number of particles per unit volume.
- Higher particle density leads to more frequent collisions between reactant molecules.
- More frequent collisions increase the likelihood that particles will collide with sufficient energy to react.
- As a result, increasing the pressure increases the reaction rate for gases.
Conversely:
- Decreasing the pressure spreads gas particles out, reducing collision frequency and slowing the reaction.
Examples:
- Haber process for ammonia production: \( \text{N}_2 + 3\text{H}_2 \rightarrow 2\text{NH}_3 \). Increasing the pressure increases the rate at which ammonia forms.
- Carbon monoxide reacting with oxygen: Increasing the pressure of CO or O₂ increases the rate of formation of CO₂.
Example
How does increasing the pressure of nitrogen and hydrogen gases affect the rate of ammonia production in the Haber process?
▶️ Answer/Explanation
- Increasing the pressure increases the number of gas molecules per unit volume.
- This causes more frequent collisions between nitrogen and hydrogen molecules.
- More collisions with sufficient energy → higher reaction rate → ammonia is formed faster.
Effect of Changing the Surface Area of Solids on Reaction Rate
The surface area of a solid reactant significantly affects the rate of reaction when it is involved in a chemical reaction. Surface area refers to how much of the solid’s surface is exposed to other reactants.
How surface area affects reaction rate:![]()
- Increasing the surface area exposes more particles of the solid to the other reactants.
- More exposed particles lead to a higher frequency of collisions between reactant particles.
- With more collisions, the probability of effective collisions (those with sufficient energy to react) increases.
- As a result, the reaction rate increases when the surface area of the solid is increased.
Conversely:
- Reducing the surface area (using large lumps instead of powder) decreases the number of exposed particles.
- Fewer collisions occur → slower reaction rate.
Examples:
- Calcium carbonate reacting with hydrochloric acid: Powdered CaCO₃ reacts faster than large pieces because more particles are exposed to acid.
- Zinc metal reacting with hydrochloric acid: Finely divided zinc powder reacts faster than a solid chunk of zinc.
Example
A student reacts calcium carbonate with hydrochloric acid using a single large piece of calcium carbonate. How would using powdered calcium carbonate affect the reaction rate and why?
▶️ Answer/Explanation
- Using powdered CaCO₃ increases the surface area of the solid exposed to the acid.
- More frequent collisions between calcium carbonate particles and HCl molecules occur.
- This increases the rate of reaction → carbon dioxide gas is produced faster.
Effect of Changing the Temperature on Reaction Rate
Temperature has a significant effect on the rate of chemical reactions because it affects the kinetic energy of particles.
How temperature affects reaction rate:![]()
- Increasing temperature increases the kinetic energy of particles.
- Particles move faster and collide more frequently.
- Higher kinetic energy means more collisions have sufficient energy to overcome the activation energy barrier.
- As a result, the reaction rate increases at higher temperatures.
Conversely:
- Lowering the temperature reduces particle movement, leading to fewer collisions.
- Fewer collisions with sufficient energy → slower reaction rate.
Examples:
- Reaction of sodium thiosulfate with hydrochloric acid: Increasing temperature makes the sulfur precipitate form faster.
- Decomposition of hydrogen peroxide (\( 2\text{H}_2\text{O}_2 \rightarrow 2\text{H}_2\text{O} + \text{O}_2 \)): Heating the solution increases the rate of oxygen production.
Effect of adding or removing a catalyst on Reaction Rate
A catalyst increases the rate of a reaction by providing an alternative reaction pathway with lower activation energy. Catalysts are not consumed in the reaction and can be reused.
![]()
Example
A student heats hydrochloric acid before reacting it with magnesium ribbon. Predict how this affects the rate of reaction and explain why.
▶️ Answer/Explanation
- Heating the acid increases the kinetic energy of HCl particles.
- Particles collide more frequently and with greater energy.
- More collisions surpass the activation energy → reaction rate increases.
- Hydrogen gas is produced faster than at room temperature.
Example
Predict how the rate of the reaction between hydrochloric acid and magnesium ribbon changes in the following situations:
- Doubling the concentration of hydrochloric acid.
- Using magnesium ribbon in powdered form instead of a strip.
- Increasing the temperature of the acid solution.
▶️ Answer/Explanation
- Doubling the concentration → more HCl particles → faster collisions → reaction rate increases.
- Using powdered magnesium → larger surface area → more particles exposed → reaction rate increases.
- Increasing temperature → particles move faster → collisions more energetic → reaction rate increases.
Example
A student investigates the reaction between hydrochloric acid and magnesium metal:
- Part A: The student uses a dilute acid solution. How will the rate of reaction change if the concentration of acid is doubled? Explain your answer.
- Part B: The student uses a magnesium strip. How would using magnesium powder instead affect the reaction rate? Explain.
- Part C: The reaction is carried out at room temperature. Predict the effect of heating the acid on the reaction rate and explain why.
- Part D: Suggest a way a catalyst could be used in a different reaction to increase its rate.
▶️ Answer/Explanation
- Part A: Doubling the concentration of hydrochloric acid increases the number of acid particles per unit volume → more frequent collisions with magnesium → rate of reaction increases.
- Part B: Using magnesium powder increases surface area → more magnesium particles exposed to collisions → rate of reaction increases compared to a strip.
- Part C: Heating the acid increases particle kinetic energy → more frequent and energetic collisions → rate of reaction increases.
- Part D: Adding a catalyst provides an alternative pathway with lower activation energy → speeds up the reaction without being consumed (e.g., manganese(IV) oxide in decomposition of hydrogen peroxide: \( 2\text{H}_2\text{O}_2 \rightarrow 2\text{H}_2\text{O} + \text{O}_2 \)).
Effect of a Catalyst on Reaction Rate
A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. It provides an alternative reaction pathway with a lower activation energy.
![]()
Key points about catalysts:
- Increases the rate of reaction by allowing more collisions to have sufficient energy to react.
- Does not alter the position of equilibrium in reversible reactions.
- Remains chemically unchanged at the end of the reaction and can be reused.
- Does not change the amounts of reactants or products, only the speed at which equilibrium is reached.
Examples:
- Manganese(IV) oxide (\( \text{MnO}_2 \)) catalyzes the decomposition of hydrogen peroxide: \( 2\text{H}_2\text{O}_2 \rightarrow 2\text{H}_2\text{O} + \text{O}_2 \)
- Platinum or nickel catalysts in the Haber process: \( \text{N}_2 + 3\text{H}_2 \rightarrow 2\text{NH}_3 \)
Example
Explain the role of manganese(IV) oxide in the decomposition of hydrogen peroxide and whether it is consumed in the reaction.
▶️ Answer/Explanation
- \( \text{MnO}_2 \) provides an alternative pathway for the decomposition with lower activation energy.
- This increases the rate of oxygen gas production from hydrogen peroxide.
- \( \text{MnO}_2 \) is not chemically changed in the reaction and can be reused.
Practical Methods for Investigating the Rate of a Reaction
Chemists can measure the rate of a reaction in different ways depending on the reactants and products involved. Two common practical methods are:
1. Measuring the Change in Mass of a Reactant or Product
![]()
- This method is suitable when a reactant is a solid that decreases in mass during the reaction or when a gas escapes from the reaction mixture.
- Using a balance, the mass of the reactants or products is measured at regular time intervals.
- The rate of reaction can be calculated from the change in mass over time.
Example:
Magnesium ribbon reacting with hydrochloric acid (\( \text{Mg} + 2\text{HCl} \rightarrow \text{MgCl}_2 + \text{H}_2 \)) produces hydrogen gas. The mass of the reaction mixture decreases as gas escapes.
2. Measuring the Formation of a Gas
![]()
- This method is suitable when one of the products is a gas.
- Gas syringe or inverted measuring cylinder in water can be used to measure the volume of gas produced at regular time intervals.
- The rate of reaction is calculated from the change in gas volume over time.
Example:
Reaction of hydrochloric acid with calcium carbonate (\( \text{CaCO}_3 + 2\text{HCl} \rightarrow \text{CaCl}_2 + \text{H}_2\text{O} + \text{CO}_2 \)) produces carbon dioxide gas. The volume of \( \text{CO}_2 \) collected over time indicates the reaction rate.
Example
A student investigates the reaction between hydrochloric acid and magnesium using two methods: (i) measuring the change in mass, and (ii) measuring the volume of hydrogen gas formed. Explain how each method can be used to determine the rate of reaction.
▶️ Answer/Explanation
- (i) Change in mass: Measure the mass of the reaction mixture at regular time intervals. The rate is calculated as the mass lost per unit time as hydrogen gas escapes.
- (ii) Gas formation: Collect hydrogen gas in a gas syringe at regular time intervals. The rate is calculated as the volume of gas produced per unit time.
- Both methods allow plotting a graph of mass or volume versus time to determine how the reaction rate changes.
Interpreting Data and Graphs from Rate of Reaction Experiments
Data from rate of reaction experiments can be presented as tables or graphs. Interpreting these helps understand how factors like concentration, temperature, or surface area affect the reaction rate.
1. Rate of Reaction from Tables![]()
- Rate can be calculated from change in mass, volume of gas, or concentration of reactants/products over time.
- Average rate = \( \dfrac{\text{change in quantity (mass, volume, or concentration)}}{\text{time}} \)
- Example: Mass of magnesium decreases from 2.00 g to 1.50 g in 50 s → Average rate = \( \dfrac{2.00 – 1.50}{50} = 0.01\,\text{g/s} \)
2. Rate of Reaction from Graphs
- Graphs can plot mass, volume of gas, or concentration versus time.
- The slope of the tangent at any point gives the instantaneous rate of reaction.
- A steeper slope indicates a faster reaction.
- Example: In the reaction of hydrochloric acid with calcium carbonate, a graph of \( \text{CO}_2 \) volume versus time shows that initially the slope is steep (fast reaction) and then decreases as the reactants are used up.

3. Comparing Effects of Different Factors
![]()
- By plotting multiple graphs for different concentrations, temperatures, or surface areas, we can visually compare how these factors affect reaction rate.
- Higher concentration, higher temperature, or greater surface area gives steeper initial slopes → faster reaction.
Example
A student measures the volume of hydrogen gas produced when magnesium reacts with hydrochloric acid at different concentrations. The graph of volume versus time shows steeper slopes for higher concentrations. Explain what this tells you about the effect of concentration on reaction rate.
▶️ Answer/Explanation
- Steeper slopes indicate a faster reaction rate.
- Higher concentration of HCl increases the number of acid particles per unit volume → more frequent collisions with magnesium.
- This confirms that increasing concentration increases the rate of reaction.
Example
The reaction between sodium thiosulfate and hydrochloric acid is studied at room temperature. The volume of sulfur precipitate is measured over time, and the results are recorded in the table below:
| Time (s) | Volume of Gas / Amount of Product (cm³) |
| 0 | 0 |
| 10 | 5 |
| 20 | 9 |
| 30 | 12 |
| 40 | 14 |
| 50 | 15 |
Calculate the average rate of reaction for the first 20 seconds and explain how the reaction rate changes over time.
▶️ Answer/Explanation
- Average rate for first 20 s: \( \text{Rate} = \dfrac{\text{Change in volume}}{\text{Time}} = \dfrac{9 – 0}{20} = 0.45\,\text{cm³/s} \)
- The rate decreases over time because the concentration of reactants decreases as the reaction proceeds → fewer collisions occur.
- This is reflected in the table as the volume increases more slowly at later time intervals (e.g., 5 cm³ in first 10 s vs 1 cm³ from 40–50 s).
Factors Affecting the Rate of Reaction (Using Collision Theory)
According to collision theory, a chemical reaction occurs when particles collide with enough energy to overcome the activation energy.
- The rate of reaction depends on:
- How frequently particles collide.
- How many collisions have enough energy to cause a reaction.
\(\text{Rate of Reaction} \propto \text{Number of successful collisions per second}\)
(a) Changing the Concentration of Solutions
- Increasing the concentration of a solution means there are more particles per unit volume.
- This leads to more frequent collisions between reactant particles.
- Hence, the number of successful collisions per second increases, and the rate of reaction increases.
![]()
Explanation using Collision Theory:
Higher concentration → more particles → more collisions → faster reaction.
Example: \(\mathrm{HCl + Mg → MgCl_2 + H_2}\): a higher concentration of \(\mathrm{HCl}\) produces hydrogen gas more quickly.
(b) Changing the Pressure of Gases
- Increasing the pressure of a gas reduces the volume, so particles are closer together.
- This increases the frequency of collisions between reacting gas molecules.
- As a result, the rate of reaction increases.
![]()
Explanation using Collision Theory:
Higher pressure → particles closer → more collisions → faster reaction.
Example: In the Haber process, increasing pressure increases the rate of ammonia production: \(\mathrm{N_2 + 3H_2 ⇌ 2NH_3}\)
(c) Changing the Surface Area of Solids
- Breaking a solid into smaller pieces or using a powder increases its surface area.
- This means more particles are exposed and available to collide with reactant particles.
- This leads to more frequent successful collisions, increasing the rate of reaction.
![]()
Explanation using Collision Theory:
Larger surface area → more exposed particles → more collisions → faster reaction.
Example: Magnesium powder reacts with acid faster than magnesium ribbon because the powder has a greater surface area.
(d) Changing the Temperature
Increasing the temperature gives particles more kinetic energy.
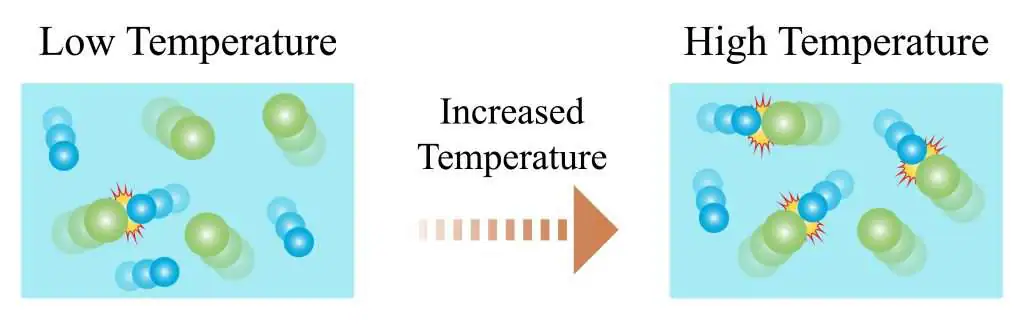
- This causes:
- More frequent collisions (particles move faster).
- A larger proportion of particles have energy ≥ activation energy.
- Therefore, the number of successful collisions per second increases, leading to a higher reaction rate.
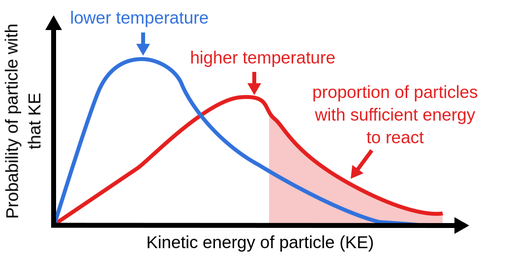
Explanation using Collision Theory:
Higher temperature → particles move faster + more have enough energy → more successful collisions → faster reaction.
Example: The decomposition of hydrogen peroxide occurs much faster when heated: \(\mathrm{2H_2O_2 → 2H_2O + O_2}\)
(e) Adding or Removing a Catalyst
A catalyst increases the rate of reaction by providing an alternative reaction pathway with a lower activation energy.
![]()
- This means more particles have enough energy to react when they collide.
- The catalyst itself is not used up during the reaction.
- Removing the catalyst would slow down the reaction.
Explanation using Collision Theory:
Catalyst → lowers activation energy → more particles can react → faster reaction.
Example: Manganese(IV) oxide catalyses the decomposition of hydrogen peroxide: \(\mathrm{2H_2O_2 → 2H_2O + O_2}\)
Factors Affecting Rate (Using Collision Theory)
| Factor | Effect on Collisions | Effect on Rate |
|---|---|---|
| ↑ Concentration (solutions) | More particles → more frequent collisions | Increases rate |
| ↑ Pressure (gases) | Particles closer → more collisions | Increases rate |
| ↑ Surface area (solids) | More surface exposed → more collisions | Increases rate |
| ↑ Temperature | Particles move faster + more have sufficient energy | Increases rate |
| Add catalyst | Lowers activation energy → more successful collisions | Increases rate |
Example Question :
Explain, in terms of collision theory, why increasing the temperature increases the rate of a chemical reaction.
▶️ Answer/Explanation
Step 1: When temperature increases, particles move faster.
Step 2: They collide more often and with greater energy.
Step 3: A greater proportion of collisions have enough energy to overcome the activation energy barrier.
Final Answer: Increasing temperature increases both the frequency and the energy of collisions, leading to a faster reaction rate.
Effect of a Catalyst on Activation Energy
A catalyst provides an alternative reaction pathway with a lower activation energy, \( E_a \), which increases the rate of reaction without being consumed in the process.
Key points:![]()
- The activation energy is the minimum energy that colliding particles must have to react.
- A catalyst lowers the \( E_a \), so more particles have sufficient energy to react at the same temperature.
- The reaction rate increases because more successful collisions occur per unit time.
- The catalyst itself is unchanged at the end of the reaction and can be reused.
Example:
- Decomposition of hydrogen peroxide: \( 2\text{H}_2\text{O}_2 \rightarrow 2\text{H}_2\text{O} + \text{O}_2 \) is slow at room temperature, but adding manganese(IV) oxide (\( \text{MnO}_2 \)) lowers the activation energy and increases the rate of oxygen production.
Example
Explain how adding a catalyst affects the activation energy and rate of decomposition of hydrogen peroxide.
▶️ Answer/Explanation
- The catalyst provides an alternative reaction pathway with lower activation energy (\( E_a \)).
- More molecules have enough energy to overcome the activation energy barrier.
- This increases the rate of reaction, producing oxygen gas faster.
- The catalyst, \( \text{MnO}_2 \), remains unchanged and can be reused.
Collision Theory: Effect of Number of Particles per Unit Volume
Collision theory explains that chemical reactions occur when reactant particles collide with sufficient energy and proper orientation. The number of particles per unit volume directly affects the frequency of collisions.
How the number of particles per unit volume affects reaction rate:
![]()
- Increasing the number of particles per unit volume increases the likelihood of collisions between reactant particles.
- More collisions per unit time lead to a higher probability of successful collisions (those with enough energy and correct orientation).
- Therefore, reactions proceed faster when the concentration (for solutions) or pressure (for gases) is higher.
Example:
- In the reaction between hydrochloric acid and sodium thiosulfate, increasing the concentration of HCl increases the number of acid particles per unit volume, causing the reaction to occur faster and the sulfur precipitate to form more quickly.
Example
Explain how increasing the concentration of a solution affects the rate of reaction according to collision theory.
▶️ Answer/Explanation
- More particles per unit volume → more frequent collisions.
- Higher probability of collisions having sufficient energy → faster reaction.
- The reaction rate increases as a result.
Effect of Frequency of Collisions Between Particles
According to collision theory, chemical reactions occur when particles collide with sufficient energy and proper orientation. The frequency of collisions significantly affects the reaction rate.
![]()
How collision frequency affects reaction rate:
- More frequent collisions increase the chance that particles will collide with enough energy to react.
- The frequency of collisions depends on factors such as:
- Concentration of reactants (more particles → more collisions)
- Pressure of gases (higher pressure → particles closer → more collisions)
- Surface area of solids (larger exposed area → more collisions at the surface)
- Higher collision frequency leads to a faster reaction rate.
Example:
- When a powdered solid reactant is used instead of a large lump, the number of collisions between reactant particles and solution molecules increases, speeding up the reaction.
Example
Why does using powdered calcium carbonate react faster with hydrochloric acid than using a single large piece, according to collision theory?
▶️ Answer/Explanation
- Powdered calcium carbonate has a larger surface area exposed to acid.
- More collisions occur per unit time between reactant particles.
- Increased frequency of collisions → higher probability of successful collisions → faster reaction.
Effect of Kinetic Energy of Particles
Collision theory states that chemical reactions occur when particles collide with sufficient energy and correct orientation. The kinetic energy of particles determines whether collisions are successful.
![]()
How kinetic energy affects reaction rate:
- Particles with higher kinetic energy move faster, increasing both the number and force of collisions.
- Only collisions where particles have energy equal to or greater than the activation energy (\( E_a \)) will lead to a reaction.
- Increasing the temperature increases the kinetic energy of particles, so a higher proportion of collisions are successful, increasing the reaction rate.
Example:
- Heating hydrochloric acid in the reaction with magnesium increases the kinetic energy of HCl particles. Faster-moving particles collide more often and with more energy, leading to quicker hydrogen gas production.
Example
Explain why the reaction between magnesium and hydrochloric acid occurs faster at higher temperatures using collision theory.
▶️ Answer/Explanation
- Higher temperature → particles have more kinetic energy.
- Particles collide more frequently and with greater energy.
- More collisions exceed the activation energy (\( E_a \)), so more successful collisions occur.
- Reaction rate increases and hydrogen gas is produced faster.
Effect of Activation Energy (E_a)
Activation energy, \( E_a \), is the minimum energy that colliding particles must have for a reaction to occur. Collision theory explains that not all collisions result in a reaction; only those with energy equal to or greater than \( E_a \) are successful.
![]()
How activation energy affects reaction rate:
- Higher activation energy means fewer collisions have enough energy to react → slower reaction.
- Lower activation energy means more collisions exceed \( E_a \) → faster reaction.
- Catalysts decrease the activation energy, providing an alternative pathway, which increases the rate of reaction.
Example:
- The decomposition of hydrogen peroxide is slow at room temperature because it has a relatively high activation energy. Adding manganese(IV) oxide lowers the \( E_a \), allowing more collisions to be successful and speeding up oxygen production: \( 2\text{H}_2\text{O}_2 \rightarrow 2\text{H}_2\text{O} + \text{O}_2 \)
Example
Explain how a catalyst increases the rate of reaction in terms of activation energy.
▶️ Answer/Explanation
- A catalyst provides an alternative reaction pathway with lower activation energy (\( E_a \)).
- More particles collide with energy greater than or equal to \( E_a \) → more successful collisions.
- The reaction rate increases without the catalyst being consumed.
