CIE iGCSE Co-ordinated Sciences-C6.3 Redox- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-C6.3 Redox – Study Notes
CIE iGCSE Co-ordinated Sciences-C6.3 Redox – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
- Define redox reactions as involving simultaneous oxidation and reduction
- Define oxidation as gain of oxygen and reduction as loss of oxygen
- Identify redox reactions as reactions involving gain and loss of oxygen
- Identify oxidation and reduction in redox reactions. (Oxidation number limited to its use to name ions, e.g. iron(II), iron(III), copper(II).)
Supplement
- Define oxidation in terms of:
(a) loss of electrons
(b) an increase in oxidation number (determination of oxidation numbers is not required) - Define reduction in terms of:
(a) gain of electrons
(b) a decrease in oxidation number (determination of oxidation numbers is not required)
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Redox Reactions
A redox reaction is a chemical reaction in which oxidation and reduction occur simultaneously. The term “redox” comes from combining reduction and oxidation.
Explanation:
- In any redox reaction, one substance is oxidised (loses electrons) while another is reduced (gains electrons).
- Therefore, oxidation and reduction always occur together — when one substance loses electrons, another must gain them.
Key idea: \(\mathrm{Oxidation \; = \; loss \; of \; electrons}\)
\(\mathrm{Reduction \; = \; gain \; of \; electrons}\)
Oxidation and Reduction in Terms of Electrons:
| Process | Definition | Example Equation |
|---|---|---|
| Oxidation | Loss of electrons | \(\mathrm{Zn → Zn^{2+} + 2e^-}\) |
| Reduction | Gain of electrons | \(\mathrm{Cu^{2+} + 2e^- → Cu}\) |
Example of a Redox Reaction:
\(\mathrm{Zn + CuSO_4 → ZnSO_4 + Cu}\)
- Oxidation: \(\mathrm{Zn → Zn^{2+} + 2e^-}\)
- Reduction: \(\mathrm{Cu^{2+} + 2e^- → Cu}\)
Explanation:
- Zinc atoms lose electrons — they are oxidised.
- Copper ions gain electrons — they are reduced.
- Both oxidation and reduction occur in the same reaction → it is a redox reaction.
Example :
Define a redox reaction and explain why the reaction between zinc and copper(II) sulfate is a redox reaction.
▶️ Answer/Explanation
Step 1: A redox reaction involves both oxidation and reduction happening together.
Step 2: In the reaction \(\mathrm{Zn + CuSO_4 → ZnSO_4 + Cu}\):
- Zinc is oxidised → \(\mathrm{Zn → Zn^{2+} + 2e^-}\)
- Copper(II) ions are reduced → \(\mathrm{Cu^{2+} + 2e^- → Cu}\)
Final Answer: It is a redox reaction because oxidation (zinc losing electrons) and reduction (copper(II) ions gaining electrons) occur simultaneously.
Oxidation and Reduction in Terms of Oxygen![]()
- Oxidation is defined as the gain of oxygen by a substance.
- Reduction is defined as the loss of oxygen by a substance.
Explanation:
- These definitions are based on reactions involving oxygen, such as combustion or metal oxide reactions.
- When a substance combines with oxygen, it is said to be oxidised.
- When a substance loses oxygen, it is said to be reduced.
- Both processes often occur together in the same reaction — making it a redox reaction.
Example 1 — Reaction of Magnesium and Copper(II) Oxide:
\(\mathrm{Mg + CuO → MgO + Cu}\)
- Magnesium gains oxygen → oxidised to magnesium oxide (\(\mathrm{MgO}\)).
- Copper(II) oxide loses oxygen → reduced to copper metal (\(\mathrm{Cu}\)).
Explanation:
- Oxidation: \(\mathrm{Mg → MgO}\)
- Reduction: \(\mathrm{CuO → Cu}\)
- Hence, both oxidation and reduction occur → it is a redox reaction.
Example 2 — Iron(III) oxide and Carbon Monoxide:
\(\mathrm{Fe_2O_3 + 3CO → 2Fe + 3CO_2}\)
- Iron(III) oxide loses oxygen → reduced to iron (\(\mathrm{Fe}\)).
- Carbon monoxide gains oxygen → oxidised to carbon dioxide (\(\mathrm{CO_2}\)).
Explanation: Reduction and oxidation occur together — making this a **redox reaction**.
Example :
In the reaction \(\mathrm{ZnO + C → Zn + CO}\), identify which substance is oxidised and which is reduced.
▶️ Answer/Explanation
Step 1: Zinc oxide (\(\mathrm{ZnO}\)) loses oxygen → it is reduced.
Step 2: Carbon gains oxygen to form carbon monoxide (\(\mathrm{CO}\)) → it is oxidised.
Final Answer: Zinc oxide is reduced, and carbon is oxidised. This is therefore a redox reaction.
Identifying Redox Reactions (in Terms of Oxygen)
A redox reaction is a chemical reaction in which both oxidation (gain of oxygen) and reduction (loss of oxygen) occur simultaneously. One substance is oxidised while another is reduced.
Key Idea:![]()
- Oxidation: Gain of oxygen.
- Reduction: Loss of oxygen.
- In a redox reaction, these two processes always happen together.
1. How to Identify Redox Reactions:
- Look for a substance that gains oxygen — it is being oxidised.
- Look for another substance that loses oxygen — it is being reduced.
- If both processes occur in the same reaction, it is a redox reaction.
2. Example 1 — Reaction Between Magnesium and Copper(II) Oxide:
\(\mathrm{Mg + CuO → MgO + Cu}\)
- Magnesium gains oxygen → it is oxidised to magnesium oxide (\(\mathrm{MgO}\)).
- Copper(II) oxide loses oxygen → it is reduced to copper metal (\(\mathrm{Cu}\)).
Conclusion: Both oxidation and reduction occur — this is a redox reaction.
3. Example 2 — Extraction of Iron Using Carbon Monoxide:
\(\mathrm{Fe_2O_3 + 3CO → 2Fe + 3CO_2}\)
- Iron(III) oxide loses oxygen → it is reduced to iron (\(\mathrm{Fe}\)).
- Carbon monoxide gains oxygen → it is oxidised to carbon dioxide (\(\mathrm{CO_2}\)).
Conclusion: This reaction involves both oxidation and reduction — it is a redox reaction.
4. Example 3 — Thermal Decomposition of Lead(II) Oxide:
\(\mathrm{PbO + CO → Pb + CO_2}\)
- \(\mathrm{PbO}\) loses oxygen → reduced to \(\mathrm{Pb}\).
- \(\mathrm{CO}\) gains oxygen → oxidised to \(\mathrm{CO_2}\).
Conclusion: Lead(II) oxide is reduced, carbon monoxide is oxidised → overall a redox reaction.
| Process | Oxygen Change | Example | Type of Reaction |
|---|---|---|---|
| Oxidation | Gain of oxygen | \(\mathrm{Mg → MgO}\) | Substance oxidised |
| Reduction | Loss of oxygen | \(\mathrm{CuO → Cu}\) | Substance reduced |
| When both occur together → Redox Reaction | |||
Example :
Identify which substance is oxidised and which is reduced in the reaction: \(\mathrm{CuO + H_2 → Cu + H_2O}\)
▶️ Answer/Explanation
Step 1: Copper(II) oxide (\(\mathrm{CuO}\)) loses oxygen → it is reduced to copper.
Step 2: Hydrogen gains oxygen → it is oxidised to water.
Final Answer: \(\mathrm{CuO}\) is reduced, \(\mathrm{H_2}\) is oxidised. Therefore, the reaction is a redox reaction.
Oxidation and Reduction in Terms of Oxidation Numbers
Redox reactions can also be identified using oxidation numbers. An oxidation number shows the charge of an ion, and it helps us track electron transfer in a reaction.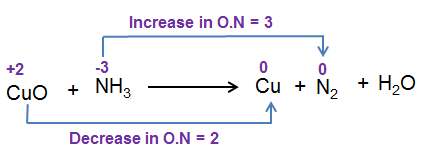
- Oxidation: Increase in oxidation number (loss of electrons).
- Reduction: Decrease in oxidation number (gain of electrons).
At the IGCSE level, oxidation numbers are mainly used to name ions such as iron(II), iron(III), copper(II), etc., and to identify changes in reactions.
Example
In the reaction:
\( \text{Fe}^{2+} \rightarrow \text{Fe}^{3+} + e^- \)
Identify what happens to iron.
▶️ Answer/Explanation
- Iron changes from \( \text{Fe}^{2+} \) to \( \text{Fe}^{3+} \).
- Oxidation number increases from +2 to +3.
- This means iron has lost an electron → iron is oxidized.
Example
In the reaction:
\( \text{Cu}^{2+} + 2e^- \rightarrow \text{Cu} \)
Identify what happens to copper.
▶️ Answer/Explanation
- Copper changes from \( \text{Cu}^{2+} \) to \( \text{Cu} \).
- Oxidation number decreases from +2 to 0.
- This means copper has gained electrons → copper is reduced.
Oxidation
Oxidation can be defined in two complementary ways. Both definitions are used to identify which species is oxidized in a chemical reaction:
(a) Oxidation as loss of electrons:
Oxidation occurs when an atom, ion, or molecule loses one or more electrons during a chemical reaction.
![]()
- Example: \( \text{Na} \rightarrow \text{Na}^+ + e^- \)
- Here, sodium loses an electron → sodium is oxidized.
(b) Oxidation as an increase in oxidation number:
Oxidation can also be described as an increase in the oxidation state (number) of an element in a compound or ion.
- Example: \( \text{Fe}^{2+} \rightarrow \text{Fe}^{3+} + e^- \)
- Oxidation number of iron increases from +2 to +3 → iron is oxidized.
Key Points:
- Oxidation always involves a transfer of electrons in some form.
- It can be identified either by actual electron loss or by observing an increase in oxidation number.
- In redox reactions, oxidation always occurs simultaneously with reduction.
Example
Identify the species being oxidized in the reaction:
\( \text{Mg} + \text{O}_2 \rightarrow 2\text{MgO} \)
▶️ Answer/Explanation
- Magnesium reacts with oxygen to form magnesium oxide.
- Magnesium loses electrons to oxygen → magnesium is oxidized.
- Oxidation number of magnesium increases from 0 to +2.
- Oxygen gains electrons (reduction) simultaneously → this is a redox reaction.
Reduction
Reduction can be defined in two complementary ways. Both definitions help identify which species is reduced in a chemical reaction:
(a) Reduction as gain of electrons:![]()
Reduction occurs when an atom, ion, or molecule gains one or more electrons during a chemical reaction.
- Example: \( \text{Cu}^{2+} + 2e^- \rightarrow \text{Cu} \)
- Copper ion gains two electrons → copper is reduced.
(b) Reduction as a decrease in oxidation number:
Reduction can also be described as a decrease in the oxidation state (number) of an element in a compound or ion.
- Example: \( \text{Fe}^{3+} + e^- \rightarrow \text{Fe}^{2+} \)
- Oxidation number of iron decreases from +3 to +2 → iron is reduced.
Key Points:
- Reduction always involves a transfer of electrons in some form.
- It can be identified either by actual electron gain or by observing a decrease in oxidation number.
- In redox reactions, reduction always occurs simultaneously with oxidation.
Example
Identify the species being reduced in the reaction:
\( \text{CuO} + \text{H}_2 \rightarrow \text{Cu} + \text{H}_2\text{O} \)
▶️ Answer/Explanation
- Copper(II) oxide loses oxygen → forms copper metal.
- Copper gains electrons during the reaction → copper is reduced.
- Hydrogen loses electrons → oxidized to water.
- Both oxidation and reduction occur simultaneously → redox reaction.
