CIE iGCSE Co-ordinated Sciences-C8.2 Group I properties- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-C8.2 Group I properties – Study Notes
CIE iGCSE Co-ordinated Sciences-C8.2 Group I properties – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
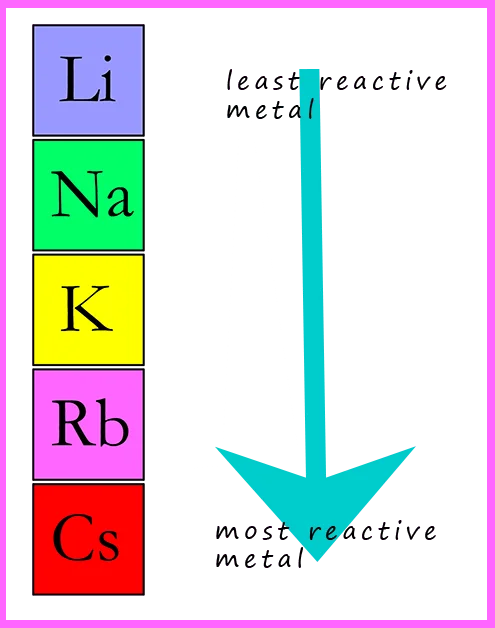
- Describe the Group I alkali metals, lithium, sodium and potassium, as relatively soft metals with general trends down the group, limited to:
(a) decreasing melting point
(b) increasing density
(c) increasing reactivity with water
Supplement
- Predict the properties of other elements in Group I, given information about the elements
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Group I Alkali Metals: Lithium, Sodium, and Potassium![]()
Group I elements, also called alkali metals, are metals found in the first column of the Periodic Table. They are characterised by having one valence electron in their outermost shell, which explains their chemical behaviour.
General Properties:
- Relatively soft metals that can be cut with a knife.
- Shiny when freshly cut but tarnish quickly in air due to reaction with oxygen.
- Good conductors of heat and electricity.
Trends Down the Group:
- Melting point: Decreases down the group. Lithium has the highest melting point, potassium the lowest. This is because metallic bonding becomes weaker as the size of the atom increases.
- Density: Increases down the group. Larger atoms pack more mass into the metallic structure, making elements like potassium denser than lithium and sodium.
- Reactivity with water: Increases down the group. Lithium reacts slowly with water, sodium more vigorously, and potassium reacts very vigorously, sometimes producing enough heat to ignite the hydrogen gas formed.

Example
Compare the reaction of lithium, sodium, and potassium with water.
▶️Answer/Explanation
Lithium reacts slowly, producing bubbles of hydrogen and forming lithium hydroxide:
\( 2\text{Li} + 2\text{H}_2\text{O} \rightarrow 2\text{LiOH} + \text{H}_2 \)
Sodium reacts more vigorously, often melting into a small ball:
\( 2\text{Na} + 2\text{H}_2\text{O} \rightarrow 2\text{NaOH} + \text{H}_2 \)
Potassium reacts very vigorously, igniting the hydrogen gas formed:
\( 2\text{K} + 2\text{H}_2\text{O} \rightarrow 2\text{KOH} + \text{H}_2 \)
Example
Explain the trend in melting points of lithium, sodium, and potassium.
▶️Answer/Explanation
Melting point decreases down the group because the metallic bonding becomes weaker. Larger atoms have more electron shells, so the single delocalised electron in the metallic lattice is further from the nucleus and less tightly held, weakening the bond between atoms.
Group I Alkali Metals: Lithium, Sodium, and Potassium
Group I elements, also called alkali metals, are metals found in the first column of the Periodic Table. They are characterised by having one valence electron in their outermost shell, which explains their chemical behaviour.
![]()
General Properties:
- Relatively soft metals that can be cut with a knife.
- Shiny when freshly cut but tarnish quickly in air due to reaction with oxygen.
- Good conductors of heat and electricity.
Trends Down the Group:
- Melting point: Decreases down the group. Lithium has the highest melting point, potassium the lowest. This is because metallic bonding becomes weaker as the size of the atom increases.
- Density: Increases down the group. Larger atoms pack more mass into the metallic structure, making elements like potassium denser than lithium and sodium.
- Reactivity with water: Increases down the group. Lithium reacts slowly with water, sodium more vigorously, and potassium reacts very vigorously, sometimes producing enough heat to ignite the hydrogen gas formed.
Example
Compare the reaction of lithium, sodium, and potassium with water.
▶️Answer/Explanation
Lithium reacts slowly, producing bubbles of hydrogen and forming lithium hydroxide:
\( 2\text{Li} + 2\text{H}_2\text{O} \rightarrow 2\text{LiOH} + \text{H}_2 \)
Sodium reacts more vigorously, often melting into a small ball:
\( 2\text{Na} + 2\text{H}_2\text{O} \rightarrow 2\text{NaOH} + \text{H}_2 \)
Potassium reacts very vigorously, igniting the hydrogen gas formed:
\( 2\text{K} + 2\text{H}_2\text{O} \rightarrow 2\text{KOH} + \text{H}_2 \)
Example
Explain the trend in melting points of lithium, sodium, and potassium.
▶️Answer/Explanation
Melting point decreases down the group because the metallic bonding becomes weaker. Larger atoms have more electron shells, so the single delocalised electron in the metallic lattice is further from the nucleus and less tightly held, weakening the bond between atoms.