CIE iGCSE Co-ordinated Sciences-C9.3 Alloys and their properties- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-C9.3 Alloys and their properties – Study Notes
CIE iGCSE Co-ordinated Sciences-C9.3 Alloys and their properties – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
- Describe alloys as mixtures of a metal with other elements, including:
(a) brass as a mixture of copper and zinc
(b) stainless steel as a mixture of iron and other elements such as chromium, nickel and carbon - State that alloys can be harder and stronger than the pure metals and are more useful
- Describe the use of alloys in terms of their physical properties, including stainless steel in cutlery because of its hardness and resistance to rusting
- Identify representations of alloys from diagrams of structure
Supplement
- Explain in terms of structure how alloys can be harder and stronger than the pure metals because the different sized atoms in alloys mean the layers can no longer slide over each other
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Alloys
An alloy is a mixture of a metal with one or more other elements, which may be metals or non-metals. Alloys are designed to have improved physical and chemical properties compared to pure metals, such as increased strength, corrosion resistance, or hardness.
![]()
Brass:
- Brass is an alloy of copper and zinc.
- The proportion of zinc can vary to adjust its properties.
- Brass is harder than pure copper, more corrosion-resistant, and has a lower melting point, making it suitable for musical instruments, decorative items, and plumbing fittings.
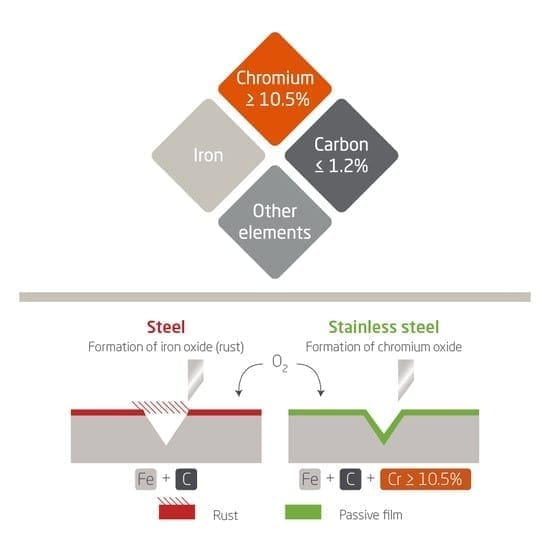
Stainless steel:
- Stainless steel is an alloy of iron with elements such as chromium, nickel, and sometimes carbon.
- Chromium gives corrosion resistance by forming a protective oxide layer on the surface, nickel improves toughness and resistance to deformation, and carbon can increase hardness.
- Stainless steel is widely used in cutlery, surgical instruments, and construction.
Example
Explain why brass is harder than copper.
▶️Answer/Explanation
Brass is a mixture of copper and zinc. The presence of zinc atoms distorts the copper lattice, making it more difficult for layers of atoms to slide over each other. This increases hardness compared to pure copper.
Example
Why is stainless steel used for kitchen knives instead of pure iron?
▶️Answer/Explanation
Stainless steel contains chromium, which forms a protective oxide layer that prevents corrosion, and nickel, which improves strength and toughness. Pure iron rusts easily and is too soft for knives, so stainless steel is preferred for durability and hygiene.
Why Alloys Are Harder and Stronger Than Pure Metals
Alloys are mixtures of metals with other elements (either metals or non-metals) that are engineered to improve their physical and mechanical properties. One of the key advantages of alloys is that they are often harder, stronger, and more durable than the pure metals from which they are made. This makes alloys more useful in a wide range of industrial, construction, and everyday applications.
![]()
Structural Explanation:
- Pure metals: In pure metals, atoms are arranged in a regular metallic lattice. The layers of metal atoms can slide over each other relatively easily when a force is applied, which makes pure metals malleable and ductile but relatively soft. This sliding occurs without breaking the metallic bonds completely, so the metal can deform under stress.
- Alloys: When other elements are added to a metal to form an alloy, atoms of different sizes are incorporated into the metallic lattice. These differently sized atoms distort the regular arrangement and create irregularities in the structure.
- The presence of these irregularly sized atoms hinders the movement of the metal layers. When an external force is applied, the layers cannot slide over each other as easily, making the alloy harder and stronger than the pure metal.
- By carefully choosing which elements to add and their proportions, alloys can be engineered to have specific properties such as increased hardness, strength, toughness, corrosion resistance, or heat resistance.
Key Points:
- Alloys disrupt the uniform lattice of metals.
- Different sized atoms act as obstacles to layer movement.
- This increases hardness, strength, and durability.
- Alloys are more versatile and useful in applications where pure metals would be too soft or weak.
Example
Explain why brass is stronger and harder than pure copper.
▶️Answer/Explanation
Brass is an alloy of copper and zinc. Zinc atoms are different in size from copper atoms and distort the regular metallic lattice. This prevents the layers of copper atoms from sliding easily under stress, making brass harder, stronger, and less malleable than pure copper.
Example
Explain why stainless steel is harder and more durable than pure iron.
▶️Answer/Explanation
Stainless steel is an alloy of iron with elements like chromium, nickel, and carbon. These atoms of different sizes disrupt the iron lattice structure, preventing the layers of iron atoms from sliding easily. This structural distortion increases the hardness and strength of stainless steel. Additionally, chromium forms a protective oxide layer that makes the alloy corrosion-resistant, further enhancing its durability compared to pure iron.
Example
How does adding carbon to iron increase the hardness of steel?
▶️Answer/Explanatio
Carbon atoms are much smaller than iron atoms and occupy spaces between them in the iron lattice. This prevents the layers of iron atoms from sliding over each other easily, increasing the hardness and strength of steel compared to pure iron. The amount of carbon controls the balance between hardness and ductility in steel.
Use of Alloys Based on Physical Properties
Alloys are used in various applications because their physical properties—such as hardness, strength, and resistance to corrosion—can be tailored by combining different elements. This makes them more suitable than pure metals for many practical uses.![]()
Stainless Steel
- Contains iron mixed with chromium, nickel, and sometimes carbon.
- The chromium gives it strong resistance to rusting by forming a thin, protective oxide layer on the surface.
- Nickel adds strength and toughness.
- Properties – hard, strong (harder and stronger than pure iron), and highly resistant to rusting or corrosion.
- Uses – cutlery, cooking utensils, surgical instruments, construction materials, and machinery. Its resistance to rusting makes it especially useful where hygiene or contact with water is involved.
Brass
- Mixture of copper and zinc.
- Properties – harder and more durable than copper, corrosion-resistant, and attractive in appearance.
- Brass also has a bright gold-like appearance and good resistance to corrosion, making it useful in musical instruments, decorative items, plumbing fittings, and coins.
Bronze
- Mixture of copper and tin.
- Properties – strong, hard, and corrosion-resistant.
- Uses – statues, medals, ship propellers, and bearings.
Duralumin
- Mixture of aluminium, copper, and magnesium.
- Properties – lightweight but very strong.
- Uses – aircraft, vehicles, and other transport industries where both low weight and high strength are important.
Example
Why is stainless steel used for kitchen knives instead of pure iron?
▶️Answer/Explanation
Stainless steel contains chromium, which forms a protective layer to prevent rusting, and carbon/nickel, which increases hardness. Pure iron rusts easily and is too soft for knives, so stainless steel is chosen for its durability, strength, and resistance to corrosion.
Example
Explain why stainless steel cutlery lasts longer than aluminium cutlery.
▶️Answer/Explanation
Aluminium is lightweight and corrosion-resistant but relatively soft. Stainless steel is much harder due to its alloyed structure, and the chromium content prevents rusting. As a result, stainless steel cutlery is stronger, more durable, and retains sharp edges better than aluminium cutlery.
Representing Alloys in Diagrams
Alloys are mixtures of a metal with one or more other elements. Their physical properties, such as increased hardness and strength, arise from the structural disruption caused by the presence of different-sized atoms. Diagrams of metallic structures can help visualise this effect.
![]()
Key Features in Alloy Diagrams:
- Base metal lattice: Represented by regular spheres arranged in a repeating pattern to depict the metallic crystal structure. This shows the arrangement in a pure metal, where layers of atoms can slide over each other easily.
- Alloying atoms: Represented by differently sized or coloured spheres to indicate the presence of other elements within the metallic lattice. These atoms do not fit perfectly into the regular arrangement of the base metal.
- Lattice distortion: The insertion of alloying atoms disrupts the regular pattern, creating obstacles that hinder the sliding of atomic layers. This structural distortion explains why alloys are harder and stronger than pure metals.
- Visual indication of property change: The more irregular the arrangement shown in the diagram, the greater the resistance to deformation, illustrating the improved mechanical properties of the alloy.
Example
Diagram of brass (copper-zinc alloy).
▶️Answer/Explanation
The diagram shows copper atoms as a regular array of spheres, while zinc atoms are represented with different colours or sizes scattered throughout the lattice. The zinc atoms distort the lattice, preventing easy sliding of layers, which increases hardness and strength compared to pure copper.
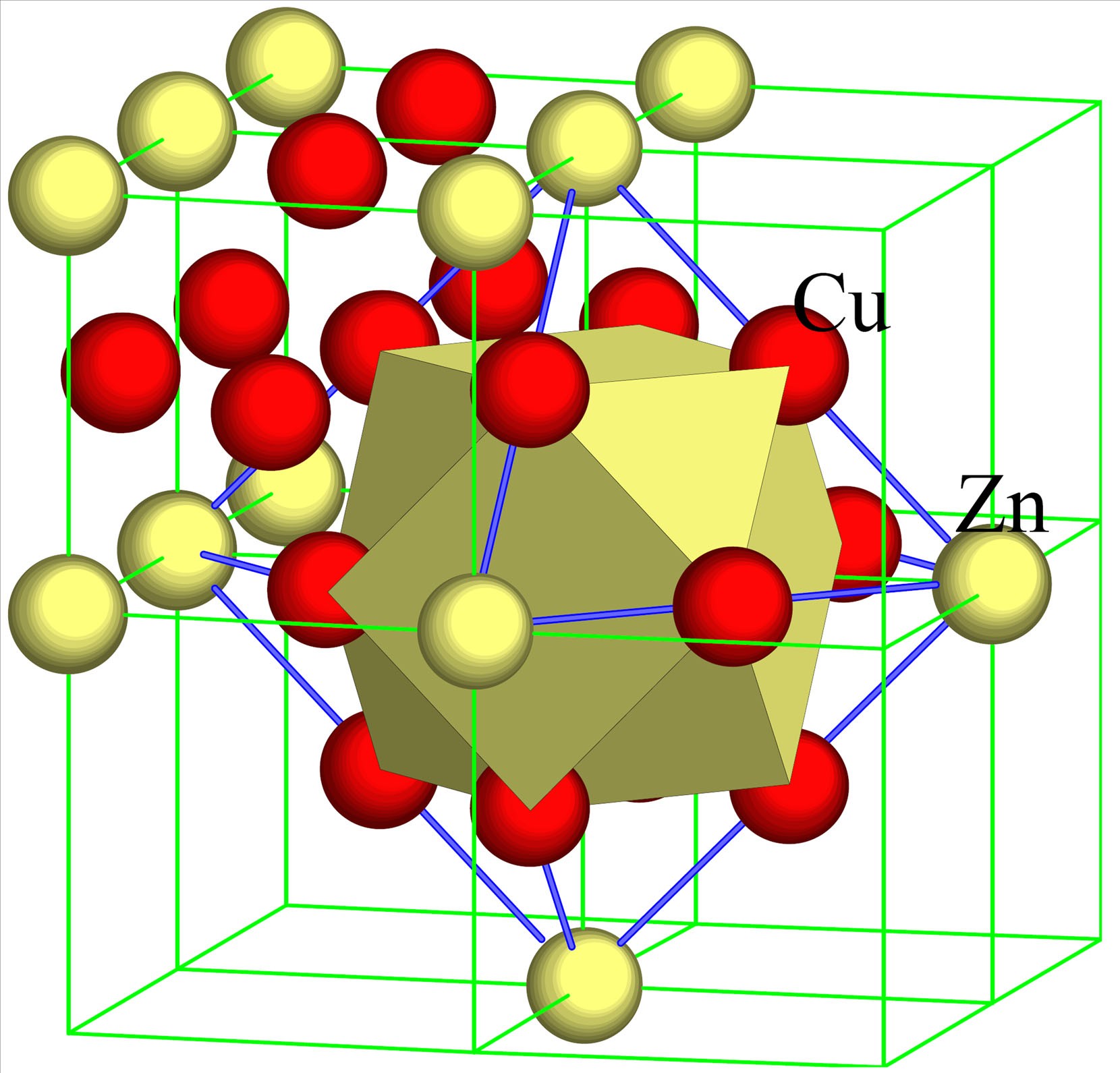
Example
Diagram of stainless steel (iron-chromium-nickel alloy).
▶️Answer/Explanation
The iron atoms form the main metallic lattice. Chromium and nickel atoms are shown as differently sized or coloured spheres inserted into the lattice. These atoms distort the regular arrangement, preventing layers of iron atoms from sliding easily. This structural feature explains why stainless steel is harder, stronger, and more resistant to corrosion than pure iron.
Why Alloys Are Harder and Stronger Than Pure Metals
An alloy is a mixture of metals (or a metal mixed with a small amount of another element). Alloys are designed to have improved properties such as increased strength, hardness, or resistance to corrosion — compared to the pure metals.
Structure of a Pure Metal:
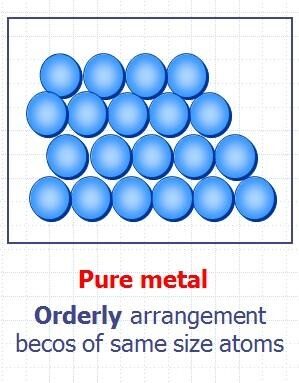
- Pure metals consist of layers of identical atoms arranged in a regular, tightly packed pattern (a metallic lattice).
- The atoms are all the same size, so the layers can slide easily over each other.
- This makes pure metals soft and malleable (can be bent and shaped easily).
Structure of an Alloy:
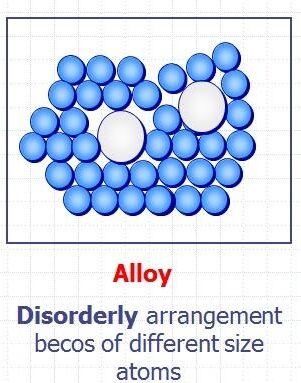
- In an alloy, the atoms of the added element are a different size compared to the metal atoms.
- This means the regular layers of atoms are distorted.
- The layers can no longer slide over each other easily.
- This makes alloys harder and stronger than pure metals.
Explanation (in terms of structure):
- In pure metals → atoms are all the same size → layers of atoms slide easily → metal is soft and malleable.
- In alloys → atoms of different sizes disrupt the regular pattern → layers cannot slide easily → alloy is harder and stronger.
Different-sized atoms in an alloy distort the regular lattice of the pure metal, making it more difficult for layers to slide past each other.
Examples of Alloys:
| Alloy | Main Components | Properties | Uses |
|---|---|---|---|
| Brass | Copper and Zinc | Harder than copper; corrosion-resistant | Musical instruments, door handles |
| Bronze | Copper and Tin | Strong and resistant to corrosion | Statues, medals, bearings |
| Steel | Iron and Carbon | Harder and stronger than iron | Bridges, buildings, tools |
Example :
Explain, in terms of structure, why alloys are harder than pure metals.
▶️ Answer/Explanation
Step 1: Pure metals have layers of identical atoms that can slide easily over each other.
Step 2: In alloys, atoms of different sizes distort the regular layers.
Step 3: This prevents the layers from sliding easily, making the alloy harder and stronger.
Final Answer: Alloys are harder than pure metals because the different sized atoms disrupt the regular structure and make it difficult for layers to slide over each other.
