CIE iGCSE Co-ordinated Sciences-P1.4 Density- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-P1.4 Density – Study Notes
CIE iGCSE Co-ordinated Sciences-P1.4 Density – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
- Define density as mass per unit volume; recall and use the equation $\rho = \frac{m}{V}$
- Describe how to determine the density of a liquid, of a regularly shaped solid and of an irregularly shaped solid which sinks in a liquid (volume by displacement), including appropriate calculations
- Determine whether an object floats or sinks based on density data
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Density
Density is the mass per unit volume of a substance.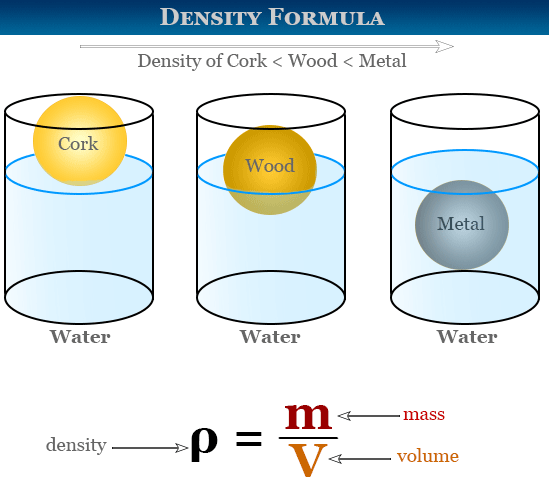
SI Unit: kilogram per cubic metre (\( \text{kg/m}^3 \)).
Equation: $\rho = \dfrac{m}{V} $
where:
- \( \rho \) = density (\( \text{kg/m}^3 \))
- \( m \) = mass (kg)
- \( V \) = volume (\( \text{m}^3 \))
Key Points:
- Density measures how tightly matter is packed inside an object.
- Different substances have different characteristic densities (e.g., metals are denser than wood).
- Objects with density less than water (\( 1000~\text{kg/m}^3 \)) float in water; objects with higher density sink.
Example:
A block of metal has a mass of \( 2.7~\text{kg} \) and a volume of \( 1.0 \times 10^{-3}~\text{m}^3 \). Calculate its density.
▶️ Answer/Explanation
Using the formula: \(\rho = \dfrac{m}{V}\)
\(\rho = \dfrac{2.7}{1.0 \times 10^{-3}}\)
\(\rho = 2700~\text{kg/m}^3\)
Therefore, the density of the metal is \( \boxed{2700~\text{kg/m}^3} \).
Determining Density of Substances
Density is defined as mass per unit volume:
$ \rho = \dfrac{m}{V} $
1. Density of a Liquid
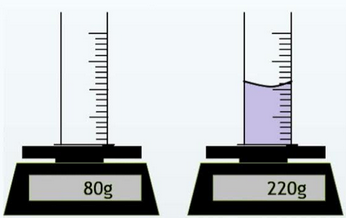
- Step 1: Place an empty measuring cylinder on a balance and note its mass.
- Step 2: Pour the liquid into the measuring cylinder and record the new mass.
- Step 3: Calculate the mass of the liquid = (mass of cylinder + liquid) − (mass of empty cylinder).
- Step 4: Read the volume of the liquid directly from the cylinder at the bottom of the meniscus.
- Step 5: Use the formula \(\rho = \dfrac{m}{V}\).
2. Density of a Regularly Shaped Solid
- Step 1: Find the mass of the solid using a balance.
- Step 2: Measure its dimensions with a ruler, vernier caliper, or micrometer.
- Step 3: Calculate its volume using the appropriate geometric formula (e.g., \( V = l \times w \times h \) for a cuboid).
- Step 4: Apply the formula \(\rho = \dfrac{m}{V}\).
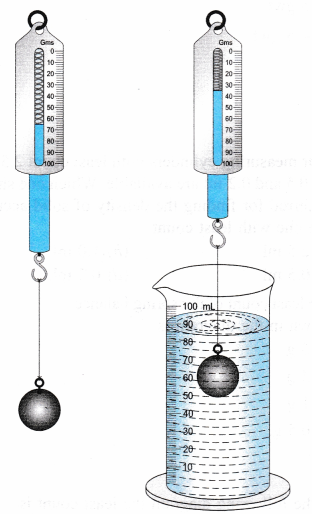
3. Density of an Irregularly Shaped Solid (Displacement Method)
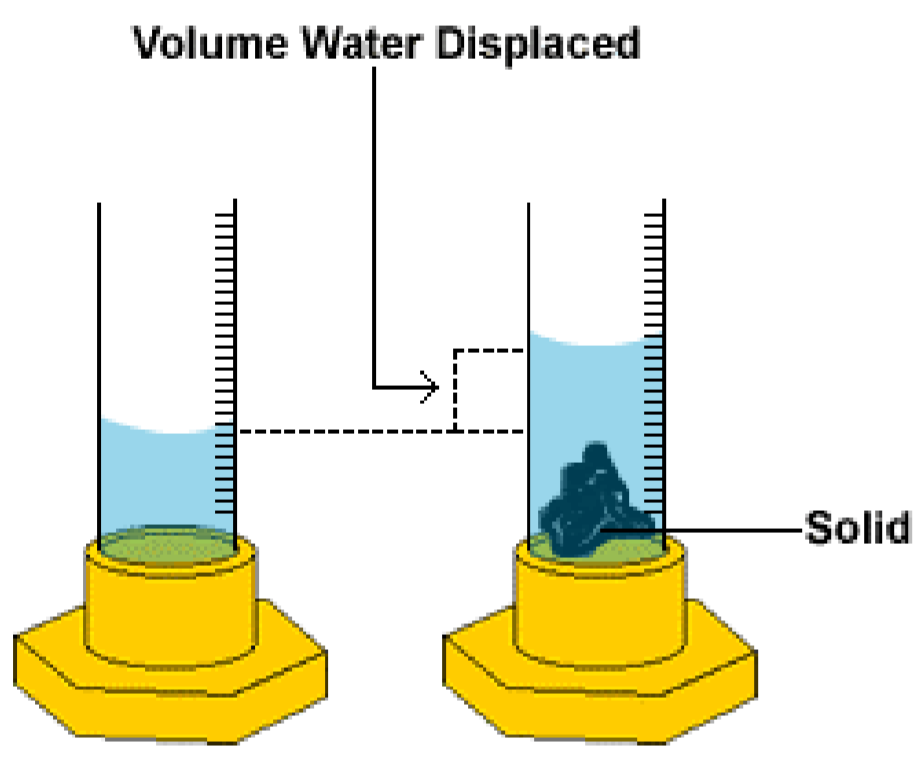
- Step 1: Measure the mass of the solid using a balance.
- Step 2: Fill a measuring cylinder (or displacement can) with water and record the initial volume \(V_1\).
- Step 3: Carefully immerse the object completely in the water and record the final volume \(V_2\).
- Step 4: Calculate the volume of the object = \( V_2 – V_1 \).
- Step 5: Use the formula \(\rho = \dfrac{m}{V}\).
Example:
A measuring cylinder has a mass of \(120~\text{g}\). When filled with oil, the total mass is \(220~\text{g}\). The cylinder shows a volume of \(100~\text{cm}^3\). Find the density of the oil.
▶️ Answer/Explanation
Mass of oil = \(220 – 120 = 100~\text{g} = 0.100~\text{kg}\)
Volume of oil = \(100~\text{cm}^3 = 1.0 \times 10^{-4}~\text{m}^3\)
Density = \(\dfrac{0.100}{1.0 \times 10^{-4}} = 1000~\text{kg/m}^3\)
So, the density of oil is \( \boxed{1000~\text{kg/m}^3} \).
Example:
A stone has a mass of \(250~\text{g}\). When placed in a measuring cylinder of water, the water level rises from \(40~\text{cm}^3\) to \(65~\text{cm}^3\). Find the density of the stone.
▶️ Answer/Explanation
Mass of stone = \(250~\text{g} = 0.250~\text{kg}\)
Volume of stone = \(65 – 40 = 25~\text{cm}^3 = 2.5 \times 10^{-5}~\text{m}^3\)
Density = \(\dfrac{0.250}{2.5 \times 10^{-5}} = 1.0 \times 10^{4}~\text{kg/m}^3\)
So, the density of the stone is \( \boxed{1.0 \times 10^{4}~\text{kg/m}^3} \).
Floating and Sinking – Density Rule
An object placed in a liquid will either float or sink depending on its density compared to the liquid.
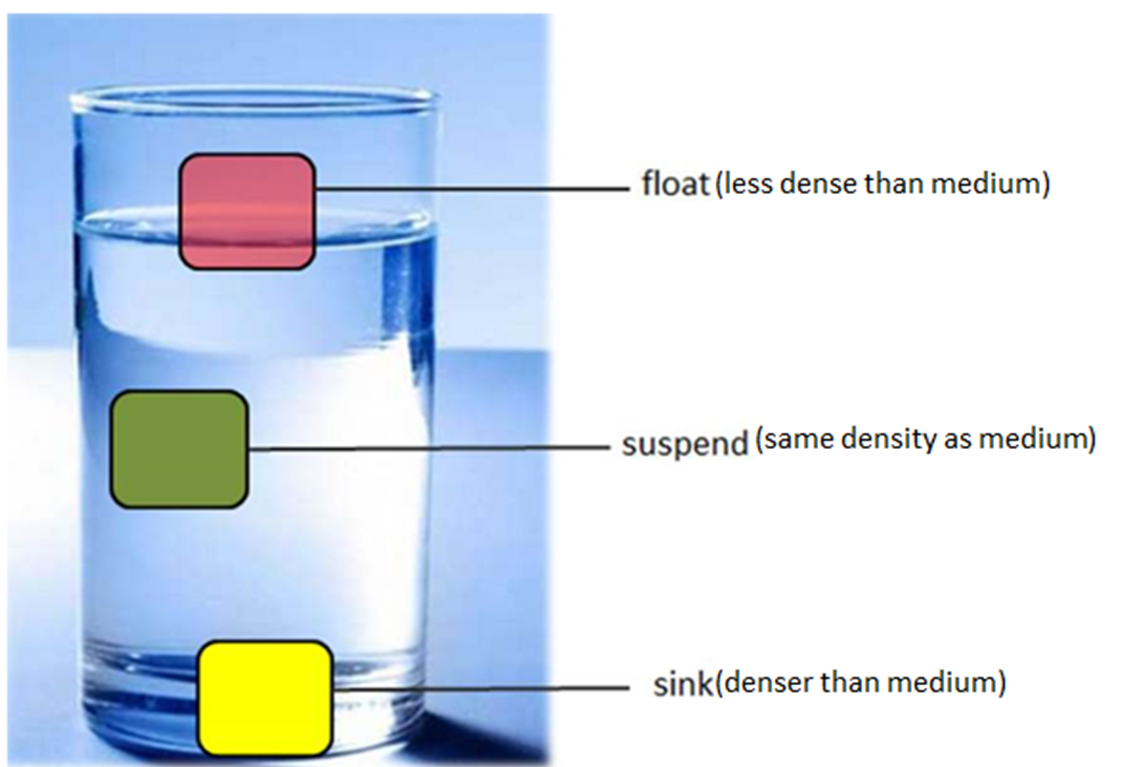
| Condition | Outcome |
|---|---|
| Object density < Liquid density | Floats (partly submerged) |
| Object density > Liquid density | Sinks |
| Object density = Liquid density | Object remains fully submerged but does not sink or rise |
Example:
A metal cube has a mass of \( 1.5~\text{kg} \) and a volume of \( 200~\text{cm}^3 \). It is placed in a liquid of density \( 1200~\text{kg/m}^3 \). Will the cube float or sink?
▶️ Answer/Explanation
Step 1: Convert volume into \( \text{m}^3 \):
\( 200~\text{cm}^3 = 200 \times 10^{-6}~\text{m}^3 = 2.0 \times 10^{-4}~\text{m}^3 \)
Step 2: Calculate density of the cube:
\[ \text{Density} = \dfrac{\text{Mass}}{\text{Volume}} = \dfrac{1.5}{2.0 \times 10^{-4}} = 7500~\text{kg/m}^3 \]
Step 3: Compare with liquid density:
Cube density = \( 7500~\text{kg/m}^3 \)
Liquid density = \( 1200~\text{kg/m}^3 \)
Since \( 7500 > 1200 \), the cube is much denser than the liquid.
Final Answer: The cube will sink to the bottom.
Example:
A block of wood has density \( 800~\text{kg/m}^3 \). It is placed in water of density \( 1000~\text{kg/m}^3 \). Will the block float or sink?
▶️ Answer/Explanation
Wood density = \( 800~\text{kg/m}^3 \)
Water density = \( 1000~\text{kg/m}^3 \)
Since \( 800 < 1000 \), the wood is less dense than water.
Therefore, the block will float, with part of it submerged.
