CIE iGCSE Co-ordinated Sciences-P1.6.1 Energy- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-P1.6.1 Energy – Study Notes
CIE iGCSE Co-ordinated Sciences-P1.6.1 Energy – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
- State that energy may be stored as kinetic, gravitational potential, chemical, elastic (strain), nuclear, electrostatic and internal (thermal).
- Describe how energy is transferred between stores during events and processes, including examples of transfer by forces (mechanical work done), electrical currents (electrical work done), heating and by electromagnetic, sound and other waves.
- Know the principle of conservation of energy and apply this principle to simple examples including the interpretation of simple flow diagrams (Sankey diagrams are not required).
Supplement
- Recall and use the equation for kinetic energy: $E_k = \frac{1}{2}mv^2$.
- Recall and use the equation for the change in gravitational potential energy: $\Delta E_p = mg\Delta h$.
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Forms of Energy Storage
Energy cannot be created or destroyed, only transferred, stored or converted from one form to another. The following are the main forms in which energy can be stored:
Kinetic Energy:
The energy an object has due to its motion. The faster an object moves, or the more massive it is, the greater its kinetic energy.
Examples: A moving car, flowing water in a river, a thrown ball.
Gravitational Potential Energy (GPE):
Energy stored in an object because of its position in a gravitational field. The higher the object, or the greater its mass, the more GPE it has.
Examples: A book on a shelf, water behind a dam, a person standing at the top of stairs.
Chemical Energy:
Energy stored in chemical bonds, released during chemical reactions. It is a major source of energy in everyday life.
Examples: Fuel (petrol, diesel), food, batteries.
Elastic (Strain) Energy:
Energy stored in an object when it is stretched, compressed, or bent, and released when the object returns to its original shape.
Examples: A stretched spring, a compressed rubber band, the bow in archery.
Nuclear Energy:
Energy stored in the nucleus of atoms, released during nuclear fission (splitting) or fusion (joining). It is an extremely powerful source of energy.
Examples: Nuclear power stations, the Sun (fusion), nuclear weapons.
Electrostatic Energy:
Energy stored because of the position of charged particles relative to each other. Like charges repel, unlike charges attract, storing potential energy in the system.
Examples: Charges in a capacitor, attraction/repulsion between charged balloons.
Internal (Thermal) Energy:
Energy stored in the microscopic motion (kinetic) and forces (potential) of particles within a substance. It increases with temperature.
Examples: Hot water, steam in a kettle, heated metal rod.
Key Point: These forms of energy are interchangeable. For example, a falling ball converts gravitational potential energy into kinetic energy. A moving car uses chemical energy from fuel and converts it into kinetic and thermal energy.
Example:
A child rides a bicycle down a hill. At the top of the hill, the child and bicycle have gravitational potential energy. As the bicycle moves down, the speed increases, and the brakes are applied to stop at the bottom.
▶️ Answer/Explanation
– At the top: The system stores gravitational potential energy due to height.
– Moving down: GPE is converted into kinetic energy as the bicycle speeds up.
– Braking: The brakes cause friction, converting kinetic energy into internal (thermal) energy in the brake pads and wheels.
– The child also uses chemical energy stored in muscles to pedal when needed.
Thus, this situation shows multiple energy stores: gravitational → kinetic → thermal, with chemical energy also involved.
Energy Transfers Between Stores
Energy cannot be created or destroyed it can only be transferred between stores or converted into other forms. The main ways energy is transferred are described below with everyday examples.
1. By Forces (Mechanical Work Done)
- When a force moves an object, work is done and energy is transferred mechanically from one store to another.
- Typical transfer: chemical energy in muscles → kinetic energy of object → thermal energy (due to friction).
- Example: Pushing a heavy box: the person’s chemical energy is turned into kinetic energy of the box and heat where friction acts.
2. By Electrical Currents (Electrical Work Done)
- Moving charges transfer energy through circuits. Electrical energy can be converted to thermal, light, mechanical, or other energy stores.
- Example: A lamp: chemical energy in a battery → electrical energy in the circuit → light + thermal energy in the filament.
3. By Heating
- Energy is transferred from a hotter object to a cooler one by conduction, convection or radiation. This increases the internal (thermal) energy of the recipient.
- Example: A hot pan transfers thermal energy to food while cooking.
4. By Waves (Electromagnetic, Sound, etc.)
- Waves carry energy without bulk transport of matter.
- Examples: Sunlight (electromagnetic) transfers energy to the Earth’s surface; sound waves transfer energy from a speaker to your ear.
Common Energy Stores (reminder)
- Kinetic energy — energy of motion.
- Gravitational potential energy — energy due to position in a gravitational field.
- Chemical energy — energy in chemical bonds (fuels, food, batteries).
- Elastic (strain) energy — energy stored in stretched/compressed objects (springs).
- Internal (thermal) energy — microscopic energy of particles (related to temperature).
- Electrostatic, nuclear and other stores.
Revision Table: Transfer methods, description and examples
| Transfer Method | Description | Everyday Example |
|---|---|---|
| Mechanical (forces) | Work done when a force moves an object; energy changes store mechanically. | Pushing a trolley; lifting a suitcase |
| Electrical currents | Charges moving in a circuit transfer electrical energy to other stores. | Kettle/Toaster/Light bulb |
| Heating | Energy flows from hot to cold; increases internal energy of cooler object. | Pan heating food, radiator warming a room |
| Waves (EM, sound) | Waves transport energy across space without moving matter. | Sunlight warming skin, sound from a speaker |
Example
An electric kettle is plugged in and switched on to boil water. Describe how energy is transferred between stores during this process.
▶️ Answer/Explanation
1. At the power plant (or battery), chemical energy (or other store) is converted and supplied as electrical energy through the mains.
2. Electrical energy flows through the kettle’s circuit and is transferred to the heating element — electrical work is done on the element.
3. The heating element’s electrical energy is converted into internal (thermal) energy of the element and then transferred by heating to the water.
4. The water’s thermal energy rises; some energy is lost to surroundings as thermal radiation and convection (dissipation).
Energy flow summary (simplified): chemical/electrical → electrical → thermal (element) → thermal (water) → surroundings.
Principle of Conservation of Energy
The principle of conservation of energy states that:
Energy cannot be created or destroyed; it can only be transferred from one store to another or converted into different forms. The total amount of energy in a closed system remains constant.
Key Notes:
- A closed system is one where no energy enters or leaves the system — energy is only transformed inside it.
- In all real processes, some energy is transferred usefully, and some is dissipated (usually as thermal energy to the surroundings).
- Energy transfers can be represented by simple flow diagrams (arrows showing useful and wasted transfers).
Common Everyday Examples:
| System / Process | Useful Transfer | Wasted Transfer |
|---|---|---|
| Electric Lamp | Electrical → Light | Electrical → Thermal (heating surroundings) |
| Car Engine | Chemical (fuel) → Kinetic (car motion) | Chemical → Thermal & Sound (wasted) |
| Food in Body | Chemical → Kinetic (movement) | Chemical → Thermal (body heat) |
Example
A pendulum bob is lifted to one side and released. Explain how the energy transfers as it swings back and forth, using the principle of conservation of energy.
▶️ Answer/Explanation
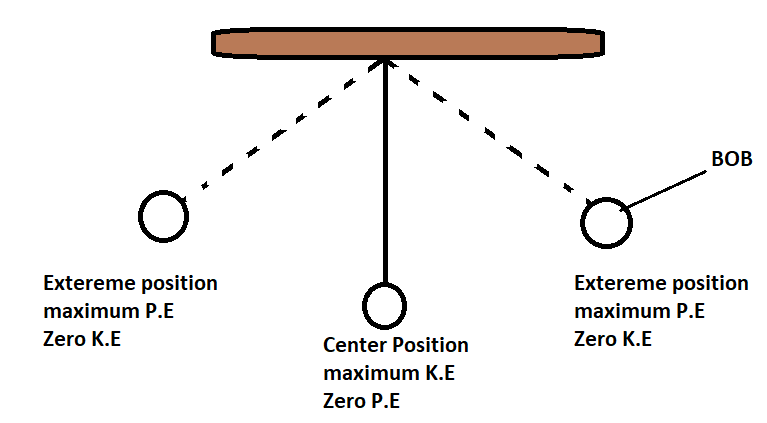
At the lowest point: kinetic energy is maximum, gravitational potential is minimum.
As it swings upwards again: kinetic energy is converted back into gravitational potential energy.
Over time, some energy is dissipated as thermal energy and sound due to air resistance and friction at the pivot, so the swing amplitude decreases.
Total energy is conserved, but useful mechanical energy decreases while wasted energy increases.
Kinetic Energy 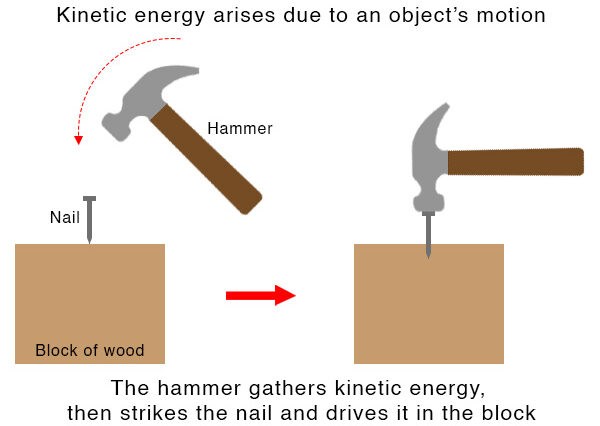
Definition: Kinetic energy is the energy an object has due to its motion. Any object with mass that is moving has kinetic energy.
Equation:
\( E_k = \dfrac{1}{2} m v^2 \)
- \( E_k \) = kinetic energy (J, joules)
- \( m \) = mass of the object (kg)
- \( v \) = speed of the object (m/s)
Key Notes:
- Kinetic energy increases with the square of the velocity — doubling the speed makes kinetic energy increase by a factor of 4.
- Heavier objects at the same speed have more kinetic energy because of greater mass.
Example:
A car of mass \( 1000~\text{kg} \) is moving at a speed of \( 20~\text{m/s} \). Calculate its kinetic energy.
▶️ Answer/Explanation
We use \( E_k = \dfrac{1}{2} m v^2 \).
\( E_k = \dfrac{1}{2} \times 1000 \times (20)^2 \)
\( E_k = 500 \times 400 \)
\( E_k = 200,000~\text{J} \)
The car has 200 kJ of kinetic energy.
Example:
A 500 kg motorcycle is moving at \( 40~\text{m/s} \). A 1000 kg car is moving at \( 20~\text{m/s} \). Which vehicle has more kinetic energy?
▶️ Answer/Explanation
We calculate kinetic energy for both:
Motorcycle:
\( E_k = \dfrac{1}{2} m v^2 = \dfrac{1}{2} \times 500 \times (40)^2 \)
\( E_k = 250 \times 1600 = 400,000~\text{J} \)
Car:
\( E_k = \dfrac{1}{2} \times 1000 \times (20)^2 \)
\( E_k = 500 \times 400 = 200,000~\text{J} \)
Conclusion: The motorcycle (smaller mass, higher speed) has twice the kinetic energy of the car. This shows how velocity has a greater effect than mass because it is squared in the formula.
Gravitational Potential Energy (GPE)
Definition: Gravitational potential energy is the energy stored in an object because of its position in a gravitational field. The higher the object is above the ground (and the more massive it is), the more gravitational potential energy it has.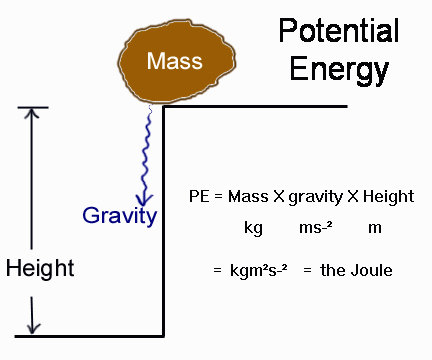
Equation:
\( E_p = m g h \)
- \( E_p \) = gravitational potential energy (J, joules)
- \( m \) = mass of the object (kg)
- \( g \) = gravitational field strength (\( 9.8~\text{N/kg} \) near Earth)
- \( h \) = height above the ground (m)
Key Notes:
- The higher the object is raised, the more GPE it gains.
- If an object falls, its GPE decreases and is usually converted into kinetic energy (ignoring air resistance).
- Mass and height directly affect GPE (it is a linear relationship).
Example:
A 2 kg book is lifted to a shelf 3 m above the ground. Calculate its gravitational potential energy relative to the ground.
▶️ Answer/Explanation
We use \( E_p = m g h \).
\( E_p = 2 \times 9.8 \times 3 \)
\( E_p = 58.8~\text{J} \)
The book gains 58.8 J of gravitational potential energy.
Example:
A 50 kg climber and a 100 kg climber both climb a mountain 100 m high. Compare their gravitational potential energies at the top.
▶️ Answer/Explanation
50 kg climber:
\( E_p = 50 \times 9.8 \times 100 = 49,000~\text{J} \)
100 kg climber:
\( E_p = 100 \times 9.8 \times 100 = 98,000~\text{J} \)
Conclusion: The heavier climber stores twice the GPE of the lighter climber because GPE is directly proportional to mass, but both climbers rise to the same height.
