CIE iGCSE Co-ordinated Sciences-P2.1.1 States of matter- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-P2.1.1 States of matter – Study Notes
CIE iGCSE Co-ordinated Sciences-P2.1.1 States of matter – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
- State the distinguishing properties of solids, liquids and gases
- Know the terms for the changes in state between solids, liquids and gases (gas to solid and solid to gas changes are not required
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
States of Matter
Matter commonly exists in three states: solid, liquid, and gas. These states are distinguished by the arrangement of their particles, the forces between them, and their observable physical properties.
Solids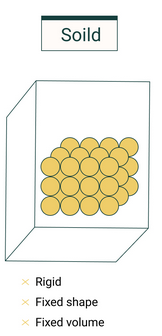
Key Features:
- Particles are arranged in a fixed, regular pattern.
- Particles vibrate about fixed positions but cannot move freely.
- Strong intermolecular forces hold particles together.
- Solids have a fixed shape and fixed volume.
- They are incompressible because particles are closely packed.
Examples: Ice, metals, wood.
Liquids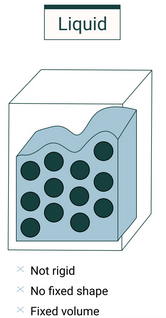
Key Features:
- Particles are closely packed, but not in a fixed arrangement.
- Particles can slide past each other, giving liquids the ability to flow.
- Intermolecular forces are weaker than in solids but stronger than in gases.
- Liquids have a fixed volume but no fixed shape (they take the shape of the container).
- They are almost incompressible.
Examples: Water, oil, mercury.
Gases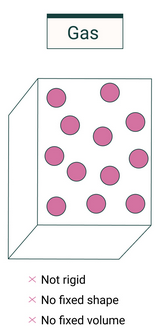
Key Features:
- Particles are far apart and arranged randomly.
- Intermolecular forces are extremely weak (almost negligible).
- Particles move rapidly in all directions with random motion.
- Gases have no fixed shape and no fixed volume; they expand to fill their container.
- They are highly compressible due to large spaces between particles.
Examples: Oxygen, carbon dioxide, hydrogen.
Key Distinguishing Properties:
| Property | Solids | Liquids | Gases |
|---|---|---|---|
| Shape | Fixed shape | Takes shape of container | No fixed shape (fills container) |
| Volume | Fixed volume | Fixed volume | No fixed volume (compressible) |
| Particle Arrangement | Closely packed, regular | Closely packed, irregular | Far apart, random |
| Particle Movement | Vibrate in fixed positions | Slide over each other | Move freely at high speeds |
| Forces Between Particles | Very strong | Moderate | Very weak |
| Compressibility | Incompressible | Almost incompressible | Highly compressible |
Changes of State:![]()
- Melting: Solid → Liquid
- Freezing: Liquid → Solid
- Boiling/Evaporation: Liquid → Gas
- Condensation: Gas → Liquid
Explanation using Particle Model:
- In solids, particles vibrate but cannot move freely due to strong intermolecular forces.
- In liquids, particles have more energy, weaker forces allow them to slide past each other.
- In gases, particles have high energy and move randomly, with negligible forces between them.
Example :
An ice cube is left at room temperature. Which change of state occurs? Explain in terms of particle arrangement.
▶️ Answer/Explanation
Step 1: The process is melting (solid → liquid).
Step 2: In the solid, particles are arranged in a fixed lattice and vibrate in place. As temperature increases, particles gain energy and can move past one another.
Final Answer: The ice melts into liquid water; particles become less ordered and move freely compared to the solid.
Example :
Steam cools down to form liquid water. Name this process and describe the energy change involved.
▶️ Answer/Explanation
Step 1: The process is condensation (gas → liquid).
Step 2: Gas particles lose kinetic energy, intermolecular forces pull them closer together, and they form a liquid.
Final Answer: Condensation occurs; thermal energy is released to the surroundings.
