CIE iGCSE Co-ordinated Sciences-P5.1 The nucleus- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-P5.1 The nucleus – Study Notes
CIE iGCSE Co-ordinated Sciences-P5.1 The nucleus – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
1. Describe the composition of the nucleus in terms of protons and neutrons
2. State the relative charges of protons, neutrons and electrons as +1, 0 and –1 respectively
3. Define the terms proton number (atomic number) Z and nucleon number (mass number) A and be able to calculate the number of neutrons in a nucleus
4. Use the nuclide notation (\mathrm{^A_Z X})
5. State that an element may have more than one isotope and know that some isotopes are radioactive
Supplement
6. Know the relationship between the proton number and the relative charge on a nucleus
7. Describe the processes of nuclear fission and nuclear fusion as the splitting and joining of nuclei
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Structure and Charge of the Nucleus
(a) Composition of the Nucleus:
- The nucleus of an atom is made up of two types of particles:
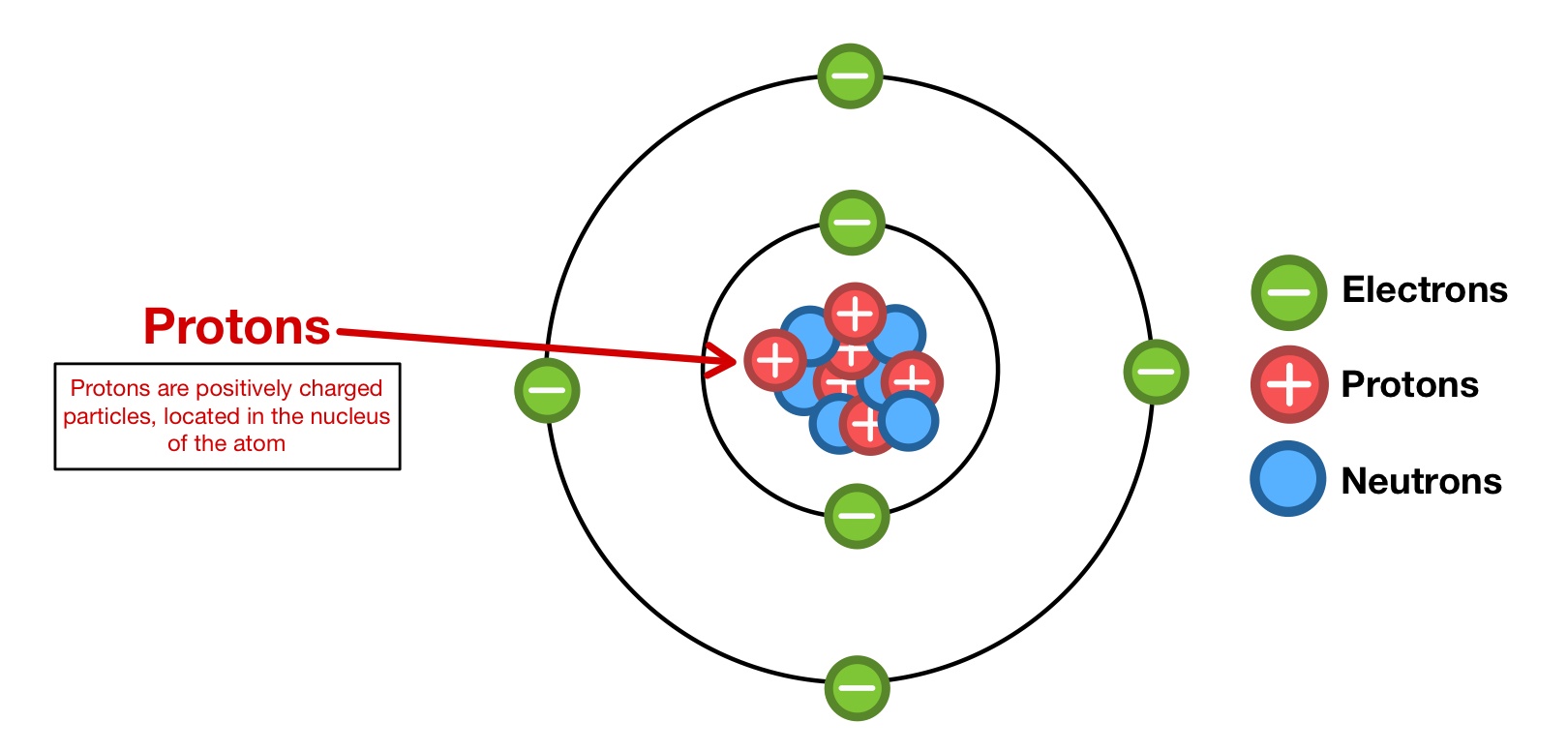
- Protons → positively charged particles.
- Neutrons → neutral particles with no charge.
- Together, protons and neutrons are called nucleons.
(b) Proton Number (Atomic Number):
- The proton number, often written as \( Z \), is the number of protons in the nucleus of an atom.
- It defines the element (e.g., hydrogen has \( Z = 1 \), carbon has \( Z = 6 \)).
- The total nuclear charge is equal to the number of protons, since each proton has charge \( +1 \).
- Therefore, the relative charge on a nucleus = \( +Z \).
(c) Relative Charges of Subatomic Particles:
- Proton: \( +1 \)
- Neutron: \( 0 \)
- Electron: \( -1 \)
(d) Summary Table of Subatomic Particles:
| Particle | Location | Relative Charge | Relative Mass |
|---|---|---|---|
| Proton | Nucleus | +1 | 1 |
| Neutron | Nucleus | 0 | 1 |
| Electron | Shells around nucleus | –1 | 1/1836 |
Example
What is the relative charge of a nucleus of a carbon atom with 6 protons and 6 neutrons?
▶️Answer/Explanation
Step (1) – Count protons:
Carbon has 6 protons, each with charge \( +1 \).
Step (2) – Neutrons:
Neutrons have charge 0, so they do not affect the total charge.
Step (3) – Total nuclear charge:
\( +6 \).
Final Answer:
The relative charge on the carbon nucleus is \( +6 \).
Nuclear Notation and Atomic Structure
(a) Proton Number (Atomic Number, Z):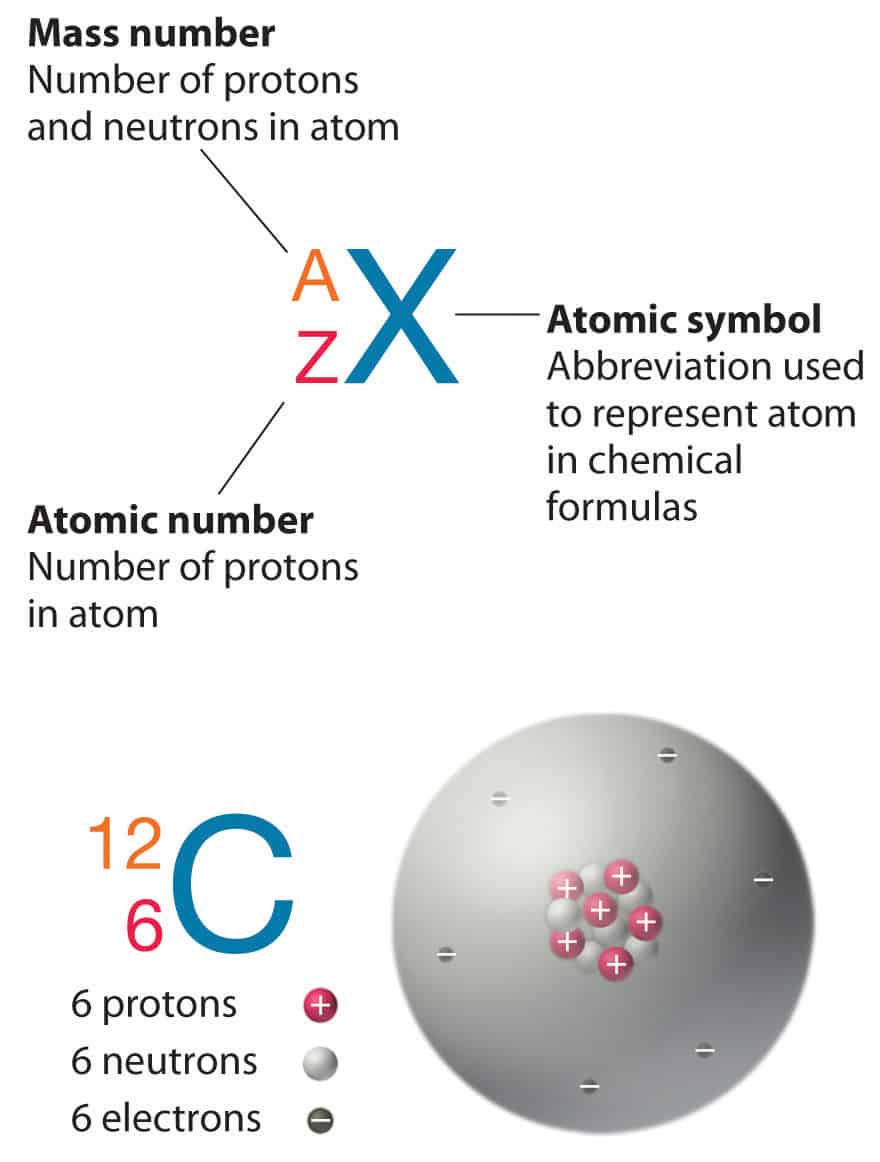
- The proton number \( Z \) is the number of protons in the nucleus of an atom.
- It determines the identity of the element (e.g., \( Z = 6 \) → Carbon, \( Z = 8 \) → Oxygen).
- The number of electrons in a neutral atom is also equal to \( Z \).
(b) Nucleon Number (Mass Number, A):
- The nucleon number \( A \) is the total number of protons and neutrons in the nucleus.
- Thus, \( A = Z + N \), where \( N \) = number of neutrons.
(c) Number of Neutrons:
- The number of neutrons can be found by:
- \( N = A – Z \).
(d) Nuclide Notation:
- An isotope is represented using the notation:
- \( _Z^A X \), where:
- \( X \) = chemical symbol of the element
- \( A \) = nucleon number (mass number)
- \( Z \) = proton number (atomic number)
Example
Chlorine has two isotopes: \( _{17}^{35}Cl \) and \( _{17}^{37}Cl \). For each isotope, calculate the number of protons, neutrons, and electrons in a neutral atom.
▶️Answer/Explanation
Step (1) – Proton number:
\( Z = 17 \) → both isotopes have 17 protons.
Step (2) – Neutron number:
For \( _{17}^{35}Cl \): \( N = A – Z = 35 – 17 = 18 \).
For \( _{17}^{37}Cl \): \( N = 37 – 17 = 20 \).
Step (3) – Electrons in neutral atom:
Equal to number of protons → 17 electrons for both isotopes.
Final Answer:
– \( _{17}^{35}Cl \): 17 protons, 18 neutrons, 17 electrons.
– \( _{17}^{37}Cl \): 17 protons, 20 neutrons, 17 electrons.
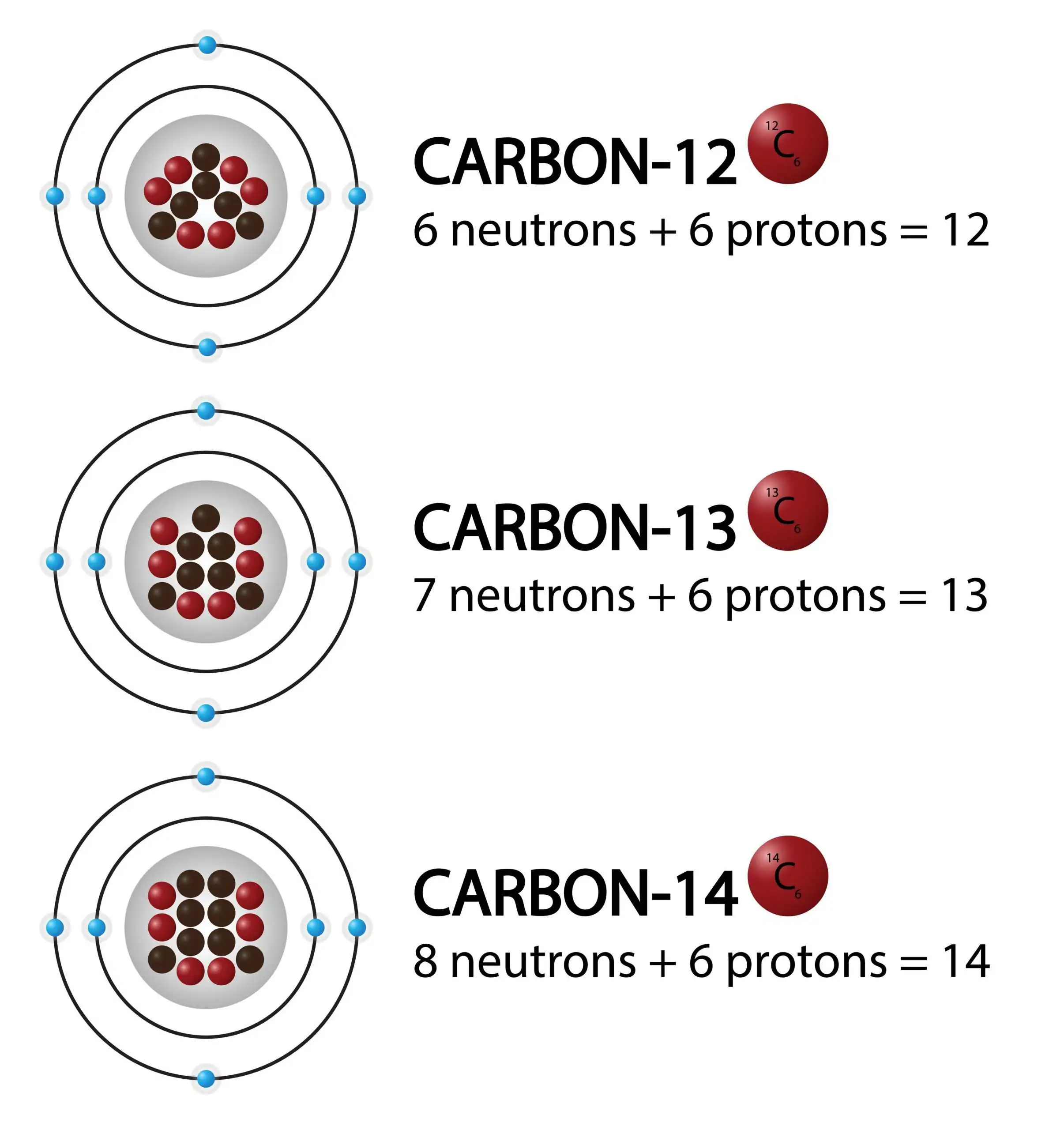
Isotopes
- Atoms of the same element can exist with different numbers of neutrons in the nucleus.

- Such atoms are called isotopes of the element.
- All isotopes of an element have the same proton number (Z) but different nucleon numbers (A).
- Example: \( _6^{12}C \) and \( _6^{14}C \) are both isotopes of carbon.
Radioactive Isotopes:
- Some isotopes are unstable and their nuclei spontaneously break down (decay), emitting radiation.
- These unstable forms are called radioisotopes.
- Example: \( _6^{14}C \) (Carbon-14) is radioactive, while \( _6^{12}C \) is stable.
Key Points:
- Isotopes of the same element have the same chemical properties (since they have the same number of electrons).
- But their physical properties (such as mass, stability, or radioactivity) can differ.
Example
Oxygen has two stable isotopes, \( _8^{16}O \) and \( _8^{18}O \). Calculate the number of neutrons in each isotope.
▶️Answer/Explanation
Step (1) – Recall formula:
\( N = A – Z \).
Step (2) – For \( _8^{16}O \):
\( N = 16 – 8 = 8 \).
Step (3) – For \( _8^{18}O \):
\( N = 18 – 8 = 10 \).
Final Answer:
– \( _8^{16}O \): 8 neutrons (stable).
– \( _8^{18}O \): 10 neutrons (stable).
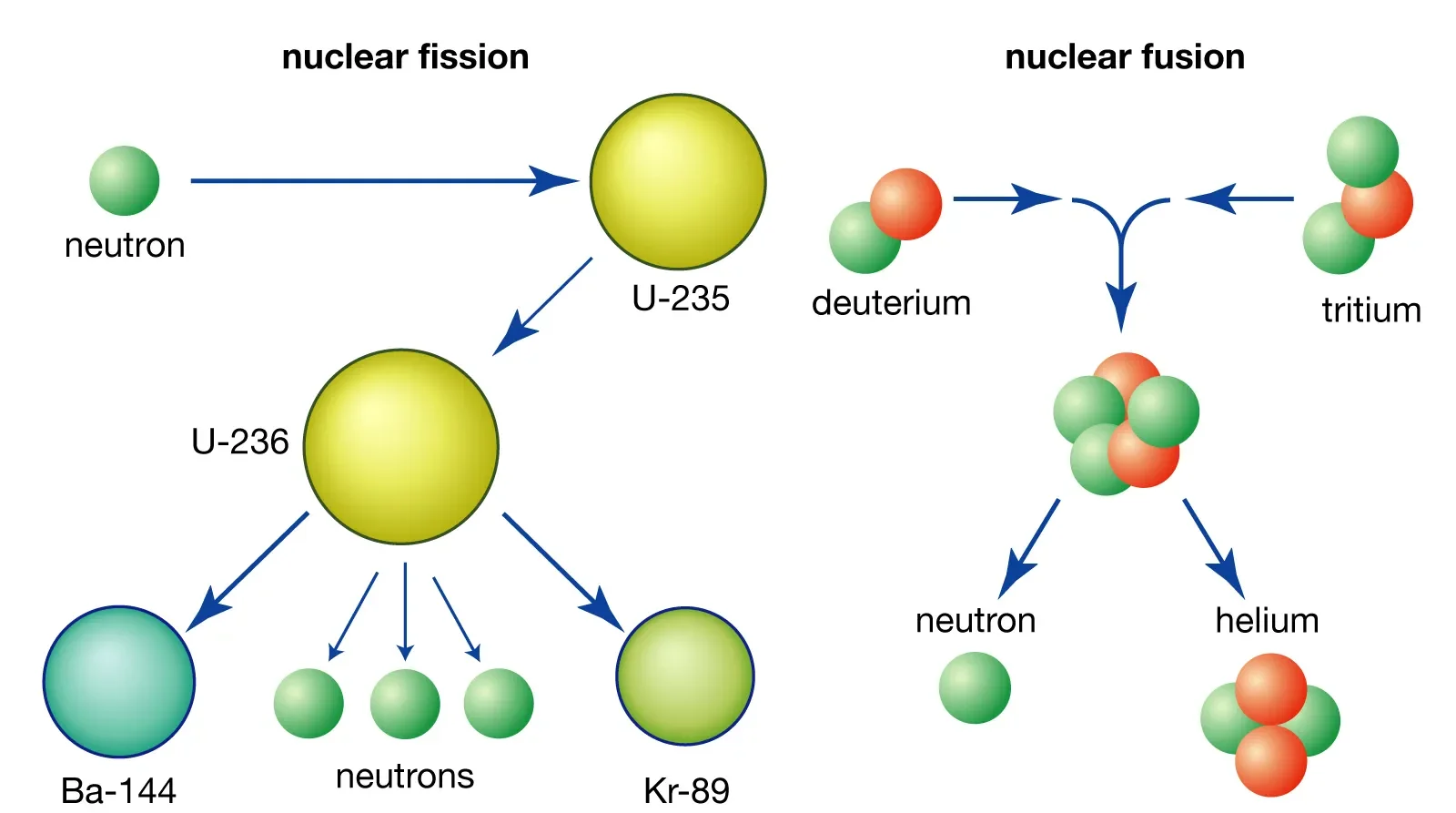
Nuclear Reactions: Fission and Fusion
(a) Nuclear Fission:
Nuclear fission is the process in which a large, unstable nucleus splits into two (or more) smaller nuclei, releasing energy.
- It is usually triggered when a heavy nucleus (such as \( ^{235}U \) or \( ^{239}Pu \)) absorbs a slow-moving neutron.
- The fission process releases:
- Two or more smaller nuclei (fission fragments)

- Several free neutrons (which may cause further fission → chain reaction)
- A large amount of energy (from the conversion of nuclear mass into energy, according to \( E = mc^2 \)).
- Two or more smaller nuclei (fission fragments)
- Applications: Used in nuclear power stations and atomic bombs.
(b) Nuclear Fusion:
Nuclear fusion is the process in which two light nuclei combine to form a larger, heavier nucleus, releasing enormous energy.
- Example: In the Sun, hydrogen nuclei fuse to form helium:
\( ^1H + ^1H \rightarrow ^2H + \text{energy} \). - Fusion requires extremely high temperatures and pressures to overcome the repulsion between positively charged nuclei.
- Applications: Powers stars and experimental fusion reactors (tokamaks, laser fusion).
| Process | Description | Example | Applications |
|---|---|---|---|
| Nuclear Fission | Splitting of a heavy nucleus into smaller nuclei | \( ^{235}U + n \rightarrow ^{141}Ba + ^{92}Kr + 3n + \text{energy} \) | Nuclear power plants, atomic bombs |
| Nuclear Fusion | Joining of light nuclei to form a heavier nucleus | \( ^2H + ^3H \rightarrow ^4He + n + \text{energy} \) | Stars (Sun), experimental reactors |
Example
In a fission reaction, \( ^{235}U \) absorbs a neutron and splits into \( ^{141}Ba \), \( ^{92}Kr \), and 3 neutrons. Calculate the total number of nucleons before and after the reaction to show conservation of nucleon number.
▶️Answer/Explanation
Step (1) – Before reaction:
\( ^{235}U + ^1n = 235 + 1 = 236 \) nucleons.
Step (2) – After reaction:
\( ^{141}Ba + ^{92}Kr + 3n = 141 + 92 + 3 = 236 \) nucleons.
Step (3) – Conservation:
Total nucleon number is the same before and after → law of conservation of nucleon number holds.
Final Answer:
236 nucleons before, 236 nucleons after → nucleon number conserved.