CIE iGCSE Co-ordinated Sciences-P5.2.2 The three types of nuclear emission- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-P5.2.2 The three types of nuclear emission – Study Notes
CIE iGCSE Co-ordinated Sciences-P5.2.2 The three types of nuclear emission – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
1. Identify alpha (α), beta (β) and gamma (γ) emissions by recalling:
(a) their nature
(b) their relative ionising effects
(c) their relative penetrating abilities
(β+ are not included, β-particles will be taken to refer to β–)
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Radioactive Emissions: Nature of α, β, and γ
(a) Nature of Emissions:
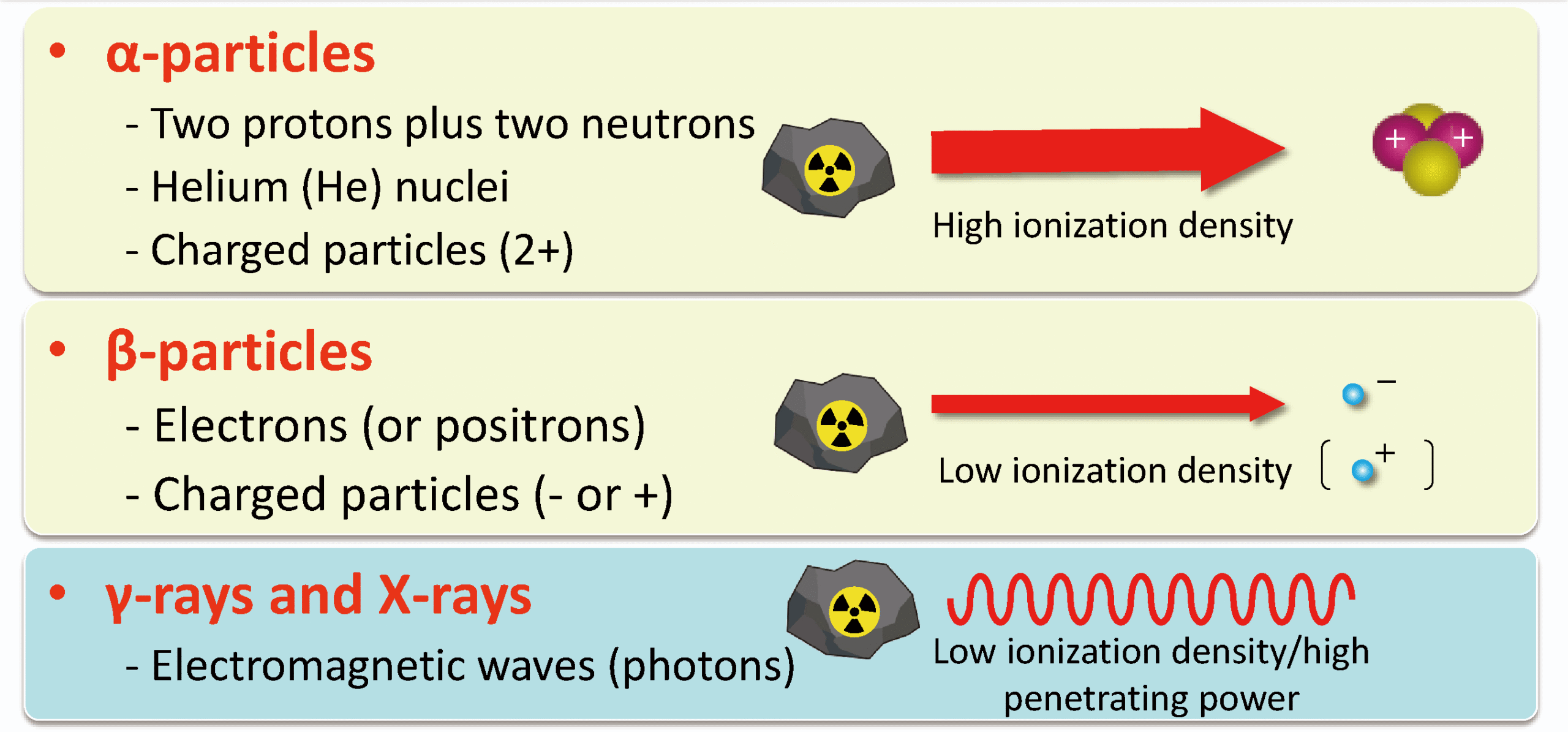
- Alpha (α): An α-particle is a helium nucleus, consisting of 2 protons and 2 neutrons. It has a mass number of 4 and a charge of +2 → \( ^4_2He \).
- Beta (β⁻): A β⁻ particle is a fast-moving electron. It is emitted from the nucleus when a neutron changes into a proton and an electron. Charge = –1, mass ≈ 0.
- Gamma (γ): A γ-ray is a high-energy electromagnetic wave (photon). It has no mass and no charge. It is often emitted after α or β decay when the nucleus is still in an excited state.
Nature of α, β, and γ:
| Type of Radiation | Symbol | Nature | Charge | Mass |
|---|---|---|---|---|
| Alpha (α) | \( ^4_2He \) | Helium nucleus (2p + 2n) | +2 | ≈ 4 u |
| Beta (β⁻) | \( e^- \) | Fast electron | –1 | ≈ 0 u |
| Gamma (γ) | γ | Electromagnetic wave (photon) | 0 | 0 |
(b) Ionising Power of α, β, and γ Radiation:
Ionisation: When radiation passes through matter, it can knock out electrons from atoms, forming ions. This is called ionisation. The ionising ability of a radiation depends on its mass, charge, and energy.
- Alpha (α):
- Strongest ionising power.
- Because α-particles are heavy (\( ^4_2He \)) and have a double positive charge (+2).
- They collide frequently with atoms, causing many ionisations in a short distance.

- Therefore, they lose energy quickly and have a very short range.
- Beta (β⁻):
- Moderate ionising power.
- Much smaller and lighter (electron, charge –1).
- Cause fewer collisions and ionisations compared to α-particles.
- Can travel further in air before losing energy.
- Gamma (γ):
- Weakest ionising power.
- No mass and no charge → interacts weakly with matter.
- Passes through materials with very little ionisation.
- However, very penetrating (can travel far before being absorbed).
Ionising Effects:
| Type of Radiation | Reason for Ionising Power | Relative Ionising Strength |
|---|---|---|
| Alpha (α) | Heavy, +2 charge, frequent collisions | Very strong |
| Beta (β⁻) | Light, –1 charge, fewer collisions | Moderate |
| Gamma (γ) | No mass, no charge, weak interactions | Very weak |
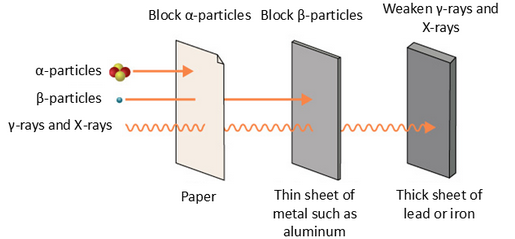
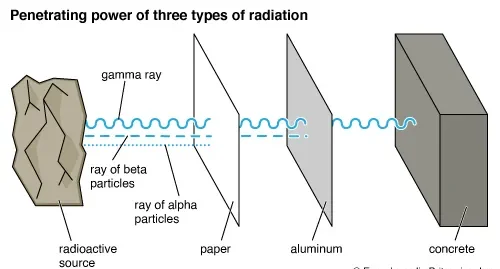
(c) Penetrating Ability of α, β, and γ Radiation:
- Alpha (α):

- Very poor penetration.
- Stopped by a thin sheet of paper or just a few cm of air.
- Cannot penetrate human skin → not dangerous outside the body.
- Danger: If inhaled or ingested, α-particles are extremely dangerous inside the body due to strong ionisation of tissues.
- Beta (β⁻):
- Moderate penetration.
- Can pass through paper but stopped by a few mm of aluminium or plastic.
- Can penetrate skin and damage tissues, so harmful both outside and inside the body.
- Gamma (γ):
- Very high penetration.
- Can travel long distances through air and pass easily through human body.
- Only significantly reduced by thick lead shielding or several metres of concrete.
- Danger: Can damage organs even when the source is outside the body.
Example
A laboratory tests three unknown radioactive sources by placing paper, aluminium, and lead between the source and a detector. The results are:
- Source X is stopped by paper.
- Source Y passes through paper but is stopped by aluminium.
- Source Z passes through both paper and aluminium, but is reduced by thick lead.
Identify the type of radiation for each source.
▶️Answer/Explanation
Step (1): If stopped by paper → must be Alpha (α).
Step (2): If stopped by aluminium → must be Beta (β⁻).
Step (3): If only reduced by lead → must be Gamma (γ).
Example
A scientist compares α, β, and γ radiation by passing them through the same gas-filled chamber. Which type of radiation will produce the most ionisation tracks, and why?
▶️Answer/Explanation
Step (1): Alpha particles are heavy and doubly charged, so they collide strongly with gas atoms.
Step (2): This causes many electrons to be knocked out → maximum ionisation per cm of travel.
Step (3): Beta produces fewer ionisations, and gamma produces the least.
Final Answer:
Alpha (α) radiation produces the most ionisation tracks because of its high mass and +2 charge.
Example
A radioactive source emits α, β, and γ radiation. Which type of radiation would:
- Be most dangerous inside the human body?
- Be most dangerous outside the body?
▶️Answer/Explanation
Step (1) – Inside the body:
Alpha particles cause very high ionisation over a short distance, damaging cells. → Most dangerous inside the body.
Step (2) – Outside the body:
Alpha particles cannot penetrate the skin, beta can penetrate slightly, but gamma rays are highly penetrating. → Most dangerous outside the body.
Final Answer:
1. Inside → Alpha (α).
2. Outside → Gamma (γ).