CIE iGCSE Co-ordinated Sciences-P5.2.3 Radioactive decay- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-P5.2.3 Radioactive decay – Study Notes
CIE iGCSE Co-ordinated Sciences-P5.2.3 Radioactive decay – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
1. Know that radioactive decay is a change in an unstable nucleus that can result in the emission of α-particles or β-particles and/or γ-radiation and know that these changes are spontaneous and random
2. Know that during α-decay or β-decay, the nucleus changes to that of a different element
3. Know the change in the nucleus that occurs during β-emission: neutron → proton + electron
Supplement
4. Use decay equations, using nuclide notation, to show the emission of α-particles, β-particles and γ-radiation
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Radioactive Decay
Radioactive decay is the process in which an unstable nucleus changes to become more stable.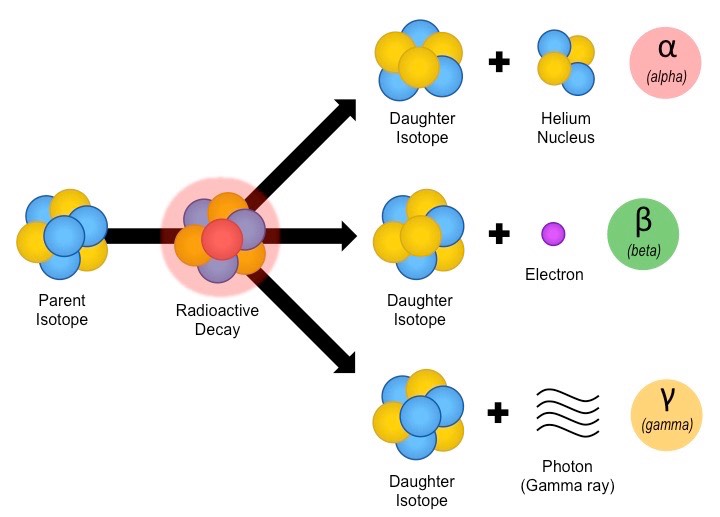
This change may result in the emission of:
- Alpha particles (α): Helium nuclei (\( ^4_2\text{He} \)) consisting of 2 protons and 2 neutrons.
- Beta particles (β⁻): High-speed electrons emitted when a neutron changes into a proton.
- Gamma radiation (γ): High-energy electromagnetic waves emitted from the nucleus.
Spontaneous: The decay occurs without being triggered by external conditions such as temperature, pressure, or chemical reactions.
Random: It is impossible to predict exactly when a particular nucleus will decay. Only the probability can be described using half-life.
Example
Which of the following statements about radioactive decay are correct?
- The decay of a nucleus depends on external temperature.
- The emission of an α-particle changes the nucleus into a new element.
- It is impossible to predict exactly when a single nucleus will decay.
▶️Answer/Explanation
Step (1): Radioactive decay is not affected by external conditions → Statement (1) is false.
Step (2): Emission of an α-particle reduces the proton number by 2 and nucleon number by 4 → new element is formed → Statement (2) is true.
Step (3): Decay is random, so we cannot predict when one nucleus will decay → Statement (3) is true.
Final Answer:
Correct statements = (2) and (3).
Nuclear Changes During α-Decay and β-Decay
When a nucleus undergoes α-decay or β-decay, the numbers of protons and neutrons in the nucleus change. Since the number of protons determines the element, the atom is transformed into a different element.
(a) Alpha (α) Decay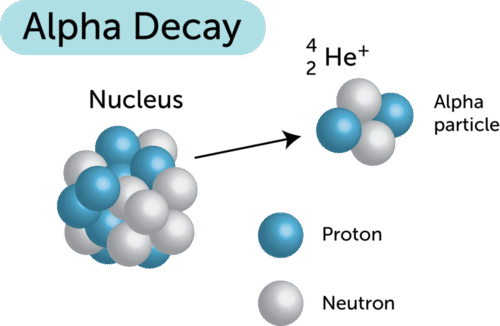
- An α-particle (\( ^4_2\text{He} \)) consisting of 2 protons and 2 neutrons is emitted.
- The parent nucleus loses 2 protons and 2 neutrons:
- Proton number (Z): decreases by 2.
- Nucleon number (A): decreases by 4.
- Result: The nucleus becomes a different element, 2 places lower in the periodic table.
Example of α-decay:
\( ^{238}_{92}\text{U} \;\;\rightarrow\;\; ^{234}_{90}\text{Th} + ^4_2\text{He} \)
Explanation: Uranium-238 decays into Thorium-234 by emitting an α-particle.
(b) Beta (β⁻) Decay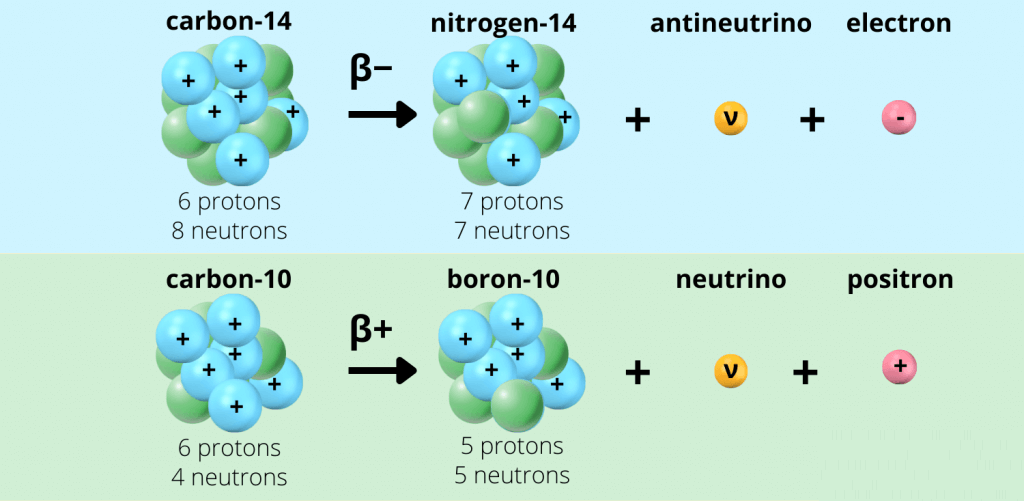
- A neutron inside the nucleus changes into a proton and an electron.
- The β⁻ particle (electron) is emitted.
- Effect on the nucleus:
- Proton number (Z): increases by 1 (a new element is formed).
- Nucleon number (A): stays the same (no nucleons lost).
- Result: The nucleus becomes a different element, 1 place higher in the periodic table.
Example of β⁻-decay:
\( ^{14}_{6}\text{C} \;\;\rightarrow\;\; ^{14}_{7}\text{N} + ^0_{-1}\text{e} \)
Explanation: Carbon-14 decays into Nitrogen-14 by emitting a β⁻ particle.
Example
A nucleus of Uranium-238 (\( ^{238}_{92}\text{U} \)) undergoes the following sequence of radioactive decays:
- It first emits one α-particle.
- The daughter nucleus then emits one β⁻-particle.
(a) Write the nuclide equation after the α-decay.
(b) Write the nuclide equation after the β⁻-decay.
(c) Identify the final element formed.
▶️Answer/Explanation
Step (1) – α-decay:
An α-particle (\( ^4_2\text{He} \)) reduces the nucleon number by 4 and the proton number by 2.
\( ^{238}_{92}\text{U} \;\;\rightarrow\;\; ^{234}_{90}\text{Th} + ^4_2\text{He} \)
So the daughter nucleus is Thorium-234 (\( ^{234}_{90}\text{Th} \)).
Step (2) – β⁻-decay:
In β⁻-decay, the nucleon number stays the same, but the proton number increases by 1.
\( ^{234}_{90}\text{Th} \;\;\rightarrow\;\; ^{234}_{91}\text{Pa} + ^0_{-1}\text{e} \)
So the daughter nucleus is Protactinium-234 (\( ^{234}_{91}\text{Pa} \)).
Step (3) – Final Answer:
(a) After α-decay → \( ^{234}_{90}\text{Th} \)
(b) After β⁻-decay → \( ^{234}_{91}\text{Pa} \)
(c) Final element formed = Protactinium
Decay Equations in Nuclide Notation: α, β, and γ Emissions
Rules for Writing Nuclear Decay Equations
- Use nuclide notation \( \;_Z^A X \;\) where \( A \) is the nucleon (mass) number and \( Z \) is the proton (atomic) number.
- Conserve nucleon number and proton number on both sides of the equation.
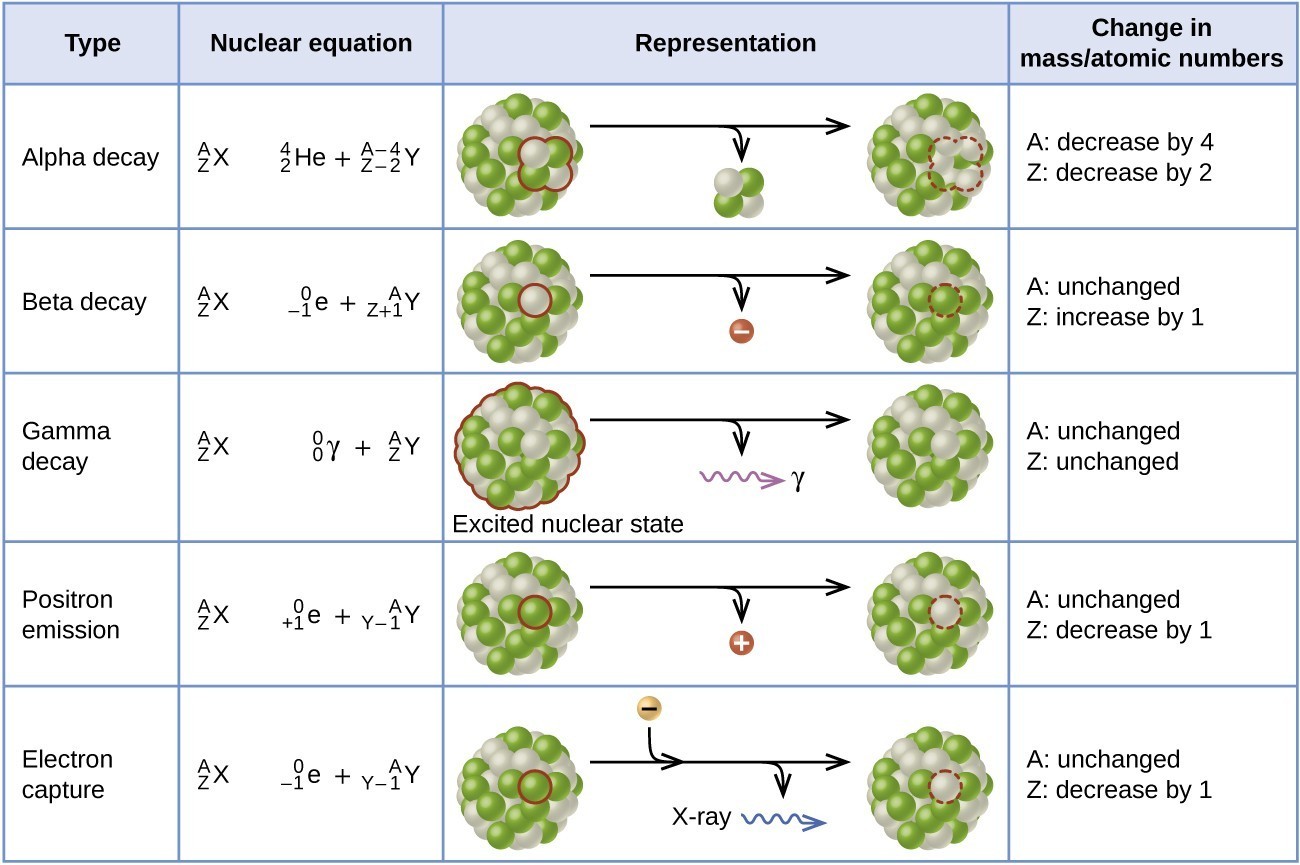
Alpha (α) Decay
- In α-decay the nucleus emits a helium nucleus \( \;_2^4\text{He} \) (2 protons, 2 neutrons).
- Change: \( A \rightarrow A-4 \), \( Z \rightarrow Z-2 \).
- General form: \( \;_Z^A X \;\rightarrow\; \;_{Z-2}^{A-4}Y \;+\; \;_2^4\text{He} \).
Example:Uranium-238 α-decay
\( \;_{92}^{238}\text{U} \;\rightarrow\; \;_{90}^{234}\text{Th} \;+\; \;_2^4\text{He} \)
Beta (β⁻) Decay
- In β⁻-decay a neutron in the nucleus becomes a proton and an electron; the electron is emitted as \( \;_{-1}^{0}\text{e} \).
- Change: \( A \rightarrow A \) (unchanged), \( Z \rightarrow Z+1 \).
- General form: \( \;_Z^A X \;\rightarrow\; \;_{Z+1}^{A}Y \;+\; \;_{-1}^{0}\text{e} \).
Example: Carbon-14 β⁻-decay
\( \;_{6}^{14}\text{C} \;\rightarrow\; \;_{7}^{14}\text{N} \;+\; \;_{-1}^{0}\text{e} \)
Gamma (γ) Emission
- γ-rays are high-energy photons emitted when a nucleus in an excited state returns to a lower energy state.
- Change: \( A \) and \( Z \) are both unchanged.
- General form: \( \;_Z^{A}X^{*} \;\rightarrow\; \;_Z^{A}X \;+\; \gamma \).
Example: Excited Cobalt-60 de-excitation
\( \;_{27}^{60}\text{Co}^{*} \;\rightarrow\; \;_{27}^{60}\text{Co} \;+\; \gamma \)
Quick Revision Table
Example
(1) Complete the α-decay equation: \( \;_{88}^{226}\text{Ra} \;\rightarrow\; \;_{?}^{?}\text{Y} \;+\; \;_2^4\text{He} \).
(2) Complete the β⁻-decay equation: \( \;_{19}^{40}\text{K} \;\rightarrow\; \;_{?}^{?}\text{Ca} \;+\; \;_{-1}^{0}\text{e} \).
(3) Write a γ-emission for excited \( \;_{53}^{131}\text{I}^{*} \).
▶️Answer/Explanation
(1) α-decay: \( A: 226 \rightarrow 226-4 = 222 \), \( Z: 88 \rightarrow 88-2 = 86 \). Element with \( Z=86 \) is Rn. \( \;_{88}^{226}\text{Ra} \rightarrow \;_{86}^{222}\text{Rn} + \;_2^4\text{He} \).
(2) β⁻-decay: \( A \) unchanged \( (=40) \), \( Z: 19 \rightarrow 19+1 = 20 \) (Ca). \( \;_{19}^{40}\text{K} \rightarrow \;_{20}^{40}\text{Ca} + \;_{-1}^{0}\text{e} \).
(3) γ-emission: \( A \) and \( Z \) unchanged; only energy is emitted. \( \;_{53}^{131}\text{I}^{*} \rightarrow \;_{53}^{131}\text{I} + \gamma \).
