CIE iGCSE Co-ordinated Sciences-P5.2.4 Half-life- Study Notes- New Syllabus
CIE iGCSE Co-ordinated Sciences-P5.2.4 Half-life – Study Notes
CIE iGCSE Co-ordinated Sciences-P5.2.4 Half-life – Study Notes -CIE iGCSE Co-ordinated Sciences – per latest Syllabus.
Key Concepts:
Core
1. Define the half-life of a particular isotope as the time taken for half the nuclei of that isotope in any sample to decay; recall and use this definition in simple calculations, which might involve information in tables or decay curves (calculations will not include background radiation)
CIE iGCSE Co-Ordinated Sciences-Concise Summary Notes- All Topics
Half-Life of a Radioactive Isotope
The half-life of an isotope is the time taken for half of the unstable nuclei in any sample to decay.
Key features:
- Half-life is a fixed property of each isotope — it does not change with temperature, pressure, or chemical bonding.
- The process of decay is random and spontaneous:
- It is impossible to predict which nucleus will decay next.
- But for a large number of nuclei, the overall decay rate follows a predictable exponential pattern.
The half-life applies equally to: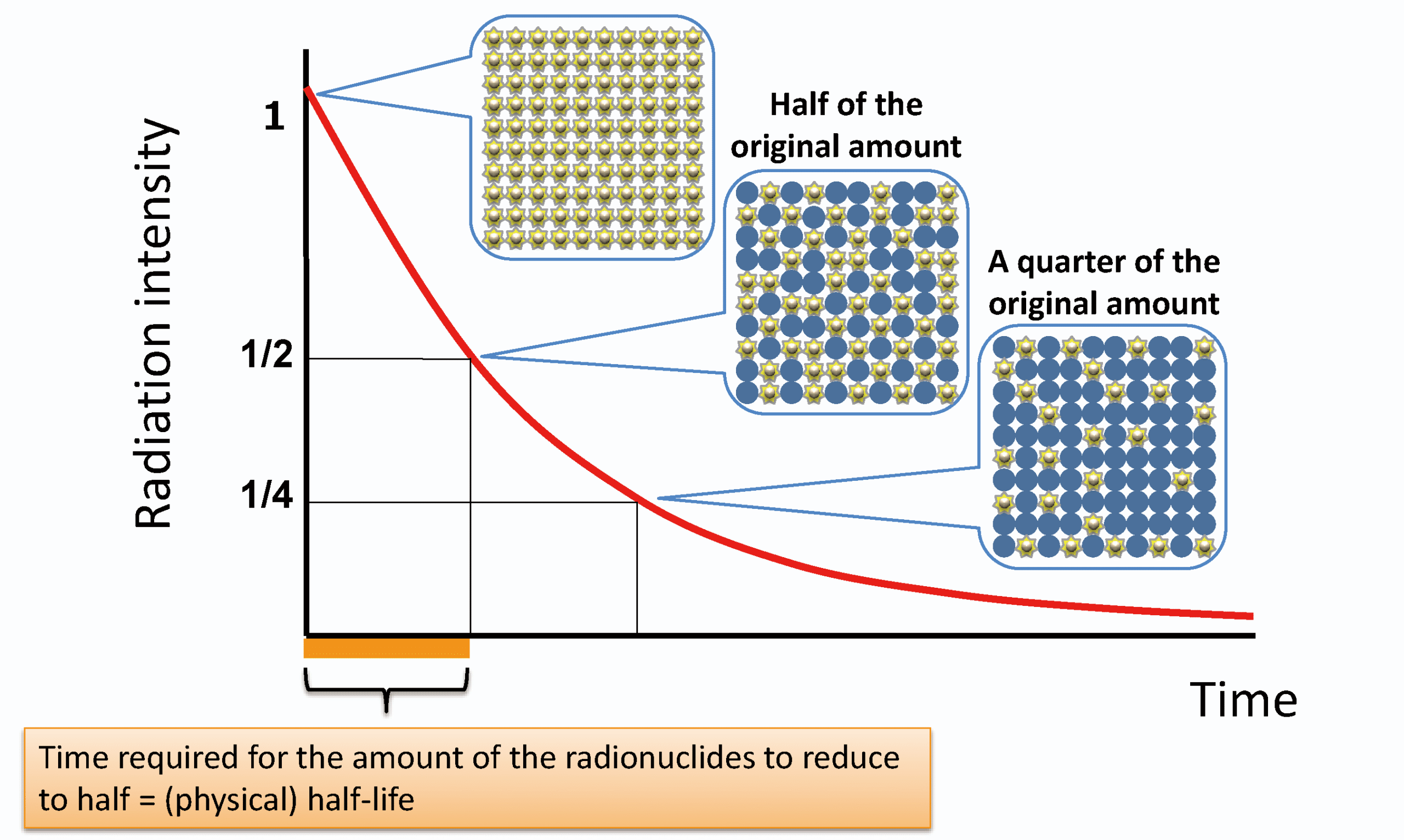
- Number of undecayed nuclei (\( N \))
- Activity (count rate, \( A \))
- Mass of radioactive material
Mathematical Representation:
The number of undecayed nuclei after time \( t \):
\( N(t) = N_0 \left( \dfrac{1}{2} \right)^{\tfrac{t}{T_{1/2}}} \)
Similarly, for activity:
\( A(t) = A_0 \left( \dfrac{1}{2} \right)^{\tfrac{t}{T_{1/2}}} \)
Extra Conceptual Notes:
- Short half-life isotopes → used in medical tracers (quickly decay, reducing long-term radiation risks).
- Long half-life isotopes → used in radioactive dating (e.g., Carbon-14 dating for archaeology, Uranium-238 dating for rocks).
- Decay curves: A graph of activity vs. time is always a smooth exponential decay. The slope gradually decreases.
Example
A radioactive isotope has a half-life of 5 days. A sample initially has an activity of 800 counts per second (cps). Calculate:
- The activity after 5 days.
- The activity after 15 days.
▶️Answer/Explanation
(a) After 1 half-life (5 days):
\( A = \dfrac{800}{2} = 400 \,\text{cps} \).
(b) After 3 half-lives (15 days):
\( A = \dfrac{800}{2^3} = \dfrac{800}{8} = 100 \,\text{cps} \).
Example
The half-life of Radium-226 is 1600 years. A sample initially contains \( N_0 = 1.6 \times 10^{24} \) atoms. Calculate the number of undecayed atoms remaining after 4800 years.
▶️Answer/Explanation
Step (1): Find the number of half-lives:
\( n = \dfrac{4800}{1600} = 3 \).
Step (2): Apply formula:
\( N = N_0 \left( \dfrac{1}{2} \right)^3 \).
\( N = 1.6 \times 10^{24} \times \dfrac{1}{8} \).
\( N = 2.0 \times 10^{23} \) atoms.
Final Answer: After 4800 years, \( 2.0 \times 10^{23} \) atoms remain undecayed.
