CIE iGCSE Biology-4.1 Biological molecules- Study Notes- New Syllabus
CIE iGCSE Biology-4.1 Biological molecules- Study Notes – New syllabus
CIE iGCSE Biology-4.1 Biological molecules- Study Notes -CIE iGCSE Biology – per latest Syllabus.
Key Concepts:
Core
- List the chemical elements that make up:
carbohydrates, fats and proteins - State that large molecules are made from smaller molecules, limited to:
(a) starch, glycogen and cellulose from glucose
(b) proteins from amino acids
(c) fats and oils from fatty acids and glycerol - Describe the use of:
(a) iodine solution test for starch
(b) Benedict’s solution test for reducing sugars
(c) biuret test for proteins
(d) ethanol emulsion test for fats and oils
(e) DCPIP test for vitamin C
Supplement
- Describe the structure of a DNA molecule:
(a) two strands coiled together to form a double helix
(b) each strand contains chemicals called bases
(c) bonds between pairs of bases hold the strands together
(d) the bases always pair up in the same way: A with T, and C with G (full names are not required)
Biological Molecules
What Are Biological Molecules?
Biological molecules are the essential building blocks of life. They make up cells, tissues, and structures in living organisms.
The three major organic molecules are:
- Carbohydrates
- Fats (Lipids)
- Proteins
Each group has a unique structure made from specific elements.
🧪 1. Carbohydrates
- Function: Main energy source for cells, used in respiration, cellulose gives structure to plant cell walls.
- Made of: Carbon (C), Hydrogen (H), Oxygen (O) – usually in a 2:1 ratio of H:O.
- Examples: Glucose, fructose (simple sugars), starch, glycogen, cellulose (complex carbs).
🧈 2. Fats (Lipids)
- Function: Long-term energy storage, insulation, protects organs, forms cell membranes.
- Made of: Carbon (C), Hydrogen (H), Oxygen (O) – more hydrogen, less oxygen than carbs.
- Examples: Butter (saturated fat), oils (unsaturated fats), cholesterol
🍗 3. Proteins
- Function: Build and repair tissues, form enzymes, hormones, muscles, transport (e.g., haemoglobin).
- Made of: Carbon (C), Hydrogen (H), Oxygen (O), Nitrogen (N), and sometimes Sulfur (S).
- Proteins are made of amino acids linked into polypeptides.
- Examples: Enzymes (amylase), structural proteins (keratin), muscle proteins (actin, myosin).
📊 Summary Table
| Molecule | Main Elements | Functions |
|---|---|---|
| Carbohydrates | C, H, O | Energy source, plant structure (cellulose) |
| Fats (Lipids) | C, H, O | Energy storage, insulation, membranes |
| Proteins | C, H, O, N (+ sometimes S) | Growth, repair, enzymes, transport |
🧠 Trick to remember the elements:
- Carbs and fats → CHO
- Proteins → CHON (and sometimes S)
Biological Molecules
Key Statement:
Large biological molecules (macromolecules) are built from smaller units (monomers) that join together through chemical reactions inside living organisms.
(a) Carbohydrates – Made from Glucose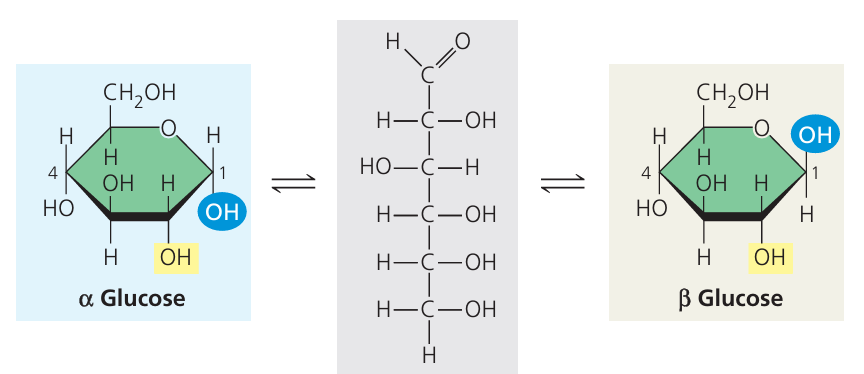
Simple sugars like glucose (a monosaccharide) join to form complex carbohydrates:
| Large Molecule | Built From | Function |
|---|---|---|
| Starch | Many glucose units | Energy storage in plants |
| Glycogen | Many glucose units | Energy storage in animals (liver & muscle) |
| Cellulose | Many glucose units | Forms plant cell walls |
🧠 Note: All made from glucose, but different bonding gives different functions.
(b) Proteins – Made from Amino Acids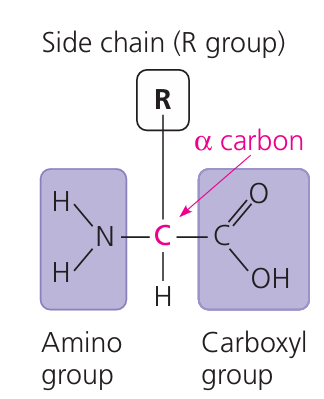
Amino acids are the building blocks of proteins. Chains of amino acids form polypeptides, which fold into functional proteins.
| Large Molecule | Built From | Function |
|---|---|---|
| Protein | Amino acids | Enzymes, hormones, muscles, cell repair |
🔧 Tip: There are 20 types of amino acids; their order determines the protein’s shape and role.
(c) Fats and Oils – Made from Fatty Acids and Glycerol
Lipids (fats and oils) are made by combining:
- 1 Glycerol molecule
- 3 Fatty acid chains
→ Together, they form a triglyceride.
| Large Molecule | Built From | Function |
|---|---|---|
| Fat/Oil (Lipid) | 3 fatty acids + 1 glycerol | Energy storage, insulation, membranes |
🧈 Note: Fats are usually solid (animals), oils are liquid (plants).
📌 Summary Table
| Macromolecule | Smaller Units (Monomers) | Example Function |
|---|---|---|
| Starch, glycogen, cellulose | Glucose (sugar units) | Energy storage, plant structure |
| Proteins | Amino acids | Enzymes, growth, repair |
| Fats and oils (lipids) | Fatty acids + Glycerol | Energy storage, membranes, insulation |
📌TIP:
- Glucose → Carbohydrates (starch, glycogen, cellulose)
- Amino acids → Proteins
- Fatty acids + Glycerol → Fats and Oils
Food Tests – Identifying Biological Molecules in Food Samples
(a) Iodine Solution Test – Detecting Starch
- Purpose: To test whether a food contains starch (a complex carbohydrate).
- Procedure: Add iodine solution to the food sample.
- Result: Starch present: Blue-black colour. No starch: Brown.
- Use Example: Rice, potato, bread.
(b) Benedict’s Solution Test – Detecting Reducing Sugars
- Purpose: To identify reducing sugars like glucose or maltose.
- Procedure: Add Benedict’s solution and heat in a water bath (~80°C).
- Result: Reducing sugar present: Blue → green → yellow → brick-red. No sugar: Remains blue.
- Use Example: Glucose in fruit juice or drinks.
- Heating is required.
(c) Biuret Test – Detecting Proteins
- Purpose: To detect the presence of proteins.
- Procedure: Add Biuret solution and shake.
- Result: Protein present: Purple/violet. No protein: Blue.
- Use Example: Milk, egg white, beans.
- Peptide bonds react with copper ions in alkaline solution.
(d) Ethanol Emulsion Test – Detecting Fats and Oils
- Purpose: To detect lipids in food.
- Procedure: Mix with ethanol, then pour into water.
- Result: Lipid present: Milky-white emulsion. No lipid: Clear solution.
- Use Example: Oils, butter, cheese.
- Lipids dissolve in ethanol but not in water → forms emulsion.
(e) DCPIP Test – Detecting Vitamin C
- Purpose: To detect vitamin C (ascorbic acid).
- Procedure: Add DCPIP solution, then food sample drop by drop.
- Result: Vitamin C present: Blue → colourless. No vitamin C: Stays blue.
- Use Example: Orange juice, lemon, vegetables.
- Fewer drops needed = more vitamin C.
📊 Summary Table – Quick Comparison
| Test | Nutrient Detected | Reagent Used | Positive Result | Heating Needed? |
|---|---|---|---|---|
| Iodine Test | Starch | Iodine solution | Blue-black | No |
| Benedict’s Test | Reducing sugars | Benedict’s solution | Brick-red precipitate | Yes |
| Biuret Test | Protein | Biuret solution | Purple/violet | No |
| Ethanol Emulsion | Fats and oils | Ethanol + water | Milky-white emulsion | No |
| DCPIP Test | Vitamin C | DCPIP solution | Blue → Colourless | No |
Structure of a DNA Molecule
Key Features of DNA Structure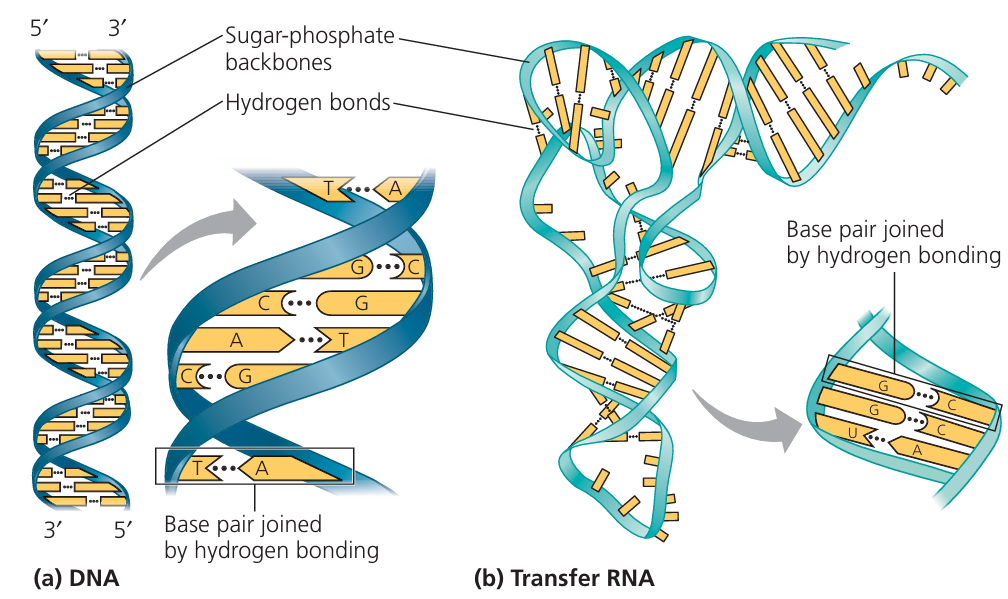
(a) Double Helix Shape
DNA consists of two long strands twisted into a double helix, resembling a spiral staircase. This coiled structure makes it compact and stable – ideal for storing genetic material inside the nucleus.
(b) Each Strand Contains Bases
Each strand of DNA is made of repeating units that include chemical bases:
A (Adenine), T (Thymine), C (Cytosine), and G (Guanine). These bases act as the code that stores genetic information.
(c) Base Pairs Hold the Strands Together
The two DNA strands are held together by weak hydrogen bonds between complementary bases. These bonds make the structure stable but allow it to unzip during replication or protein synthesis.
(d) Bases Always Pair in the Same Way
Base pairing follows strict complementary rules:
| Base on Strand 1 | Pairs With (Strand 2) |
|---|---|
| A (Adenine) | T (Thymine) |
| C (Cytosine) | G (Guanine) |
These specific pairings help ensure accurate copying of DNA during cell division.
📌 Summary Points
- DNA is a double helix – two strands twisted together.
- Each strand has chemical bases: A, T, C, and G.
- Hydrogen bonds between base pairs hold the strands together.
- Complementary base pairing: A ↔ T, and C ↔ G.
