CIE IGCSE Physics (0625) Conduction Study Notes - New Syllabus
CIE IGCSE Physics (0625) Conduction Study Notes
LEARNING OBJECTIVE
- Understanding the concepts of Conduction
Key Concepts:
- Melting and Boiling
- Condensation and Solidification
- Evaporation
- Differences Between Boiling and Evaporation
Factors Affecting the Rate of Evaporation
Good thermal conductors and Bad thermal conductors (Thermal insulators)
Experiment 1: Comparing Thermal Conductivity of Different Materials
Aim: To compare how well different materials conduct heat.
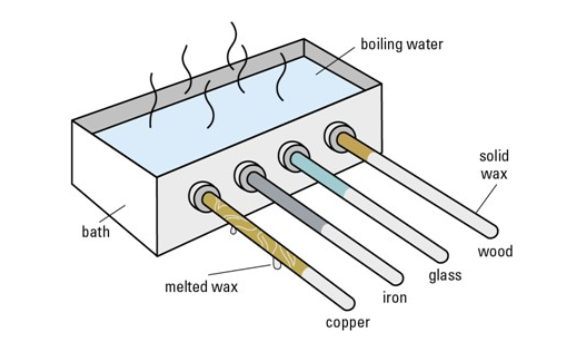
Apparatus:
- Metal rod (e.g. copper)
- Plastic rod and wooden rod (same length and thickness)
- Wax
- Drawing pins or small metal balls
- Clamp stand
- Heat source (e.g. Bunsen burner or hot water)
Method:
- Fix the rods horizontally to a clamp stand, and apply a small blob of wax with a drawing pin to the end of each rod.
- Heat the other ends of the rods at the same time using a Bunsen burner or by placing them in contact with hot water.
- Observe how long it takes for the wax to melt and the drawing pins to fall off.
Observation:
- The drawing pin on the metal rod falls off first, followed by wood, and then plastic (or maybe not at all for poor conductors).
Conclusion:
- Good thermal conductors (like metal) transfer heat quickly, melting the wax fastest.
- Bad thermal conductors (wood/plastic) transfer heat slowly, taking much longer.
Experiment 2: Comparing Thermal Insulating Ability of Different Materials

Aim: To find out which materials are good thermal insulators by observing heat loss.
Apparatus:
- Several small beakers or test tubes
- Hot water
- Thermometers
- Insulating materials (e.g. cotton wool, bubble wrap, newspaper, foil, and one with no insulation)
- Stopwatch or timer
Method:
- Wrap each beaker/test tube in a different insulating material. Leave one unwrapped as a control.
- Pour the same volume of hot water (e.g. 80°C) into each container and insert thermometers.
- Record the starting temperatures.
- After a fixed time (e.g. 10 or 15 minutes), record the final temperatures.
- Compare how much heat each beaker lost.
Observation:
- The beaker wrapped in the best insulating material (e.g. cotton wool) retains the most heat (smallest temperature drop).
- The beaker with no insulation cools the fastest.
Conclusion:
- Good thermal insulators reduce heat loss and help maintain temperature.
- Poor insulators allow heat to escape more quickly.
Thermal Conduction in Solids
Thermal Conduction in Solids
Thermal conduction is the transfer of heat through a solid without any movement of the solid itself.
1. In All Solids – Lattice Vibrations
Structure: All solids have atoms or molecules arranged in a fixed, regular pattern called a lattice.

- When one part of a solid is heated, its particles (atoms or ions) gain kinetic energy and begin to vibrate more rapidly.
- These vibrations are passed along to neighboring particles by collisions.
- This is how heat energy travels through non-metals and insulating solids.

Note: In insulators (like wood or plastic), only lattice vibrations are responsible for heat transfer, and they are slow, making these materials poor conductors.
2. In Metals – Free (Delocalised) Electrons

- When a metal is heated, both the lattice particles vibrate and the free electrons gain energy.
- These high-energy electrons move quickly through the metal, colliding with atoms and transferring energy more rapidly.
- This makes metals excellent thermal conductors.
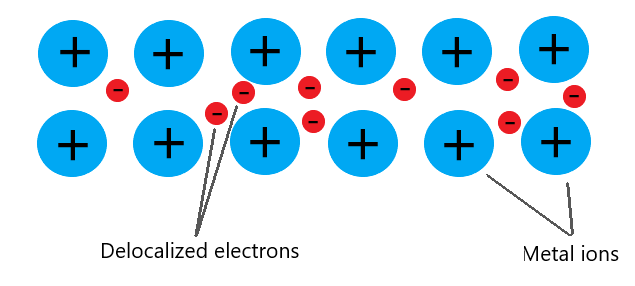
Key Point: Free electrons carry energy much faster than vibration alone. This is why metals conduct heat better than non-metals.
Poor Thermal Conduction in Gases and Most Liquids
1. Large Particle Spacing:

- In gases and most liquids, particles are not tightly packed.
- There are large spaces between particles compared to solids.
- This makes it harder for vibrating particles to transfer energy to neighboring particles by collisions.
2. Weak Intermolecular Forces:
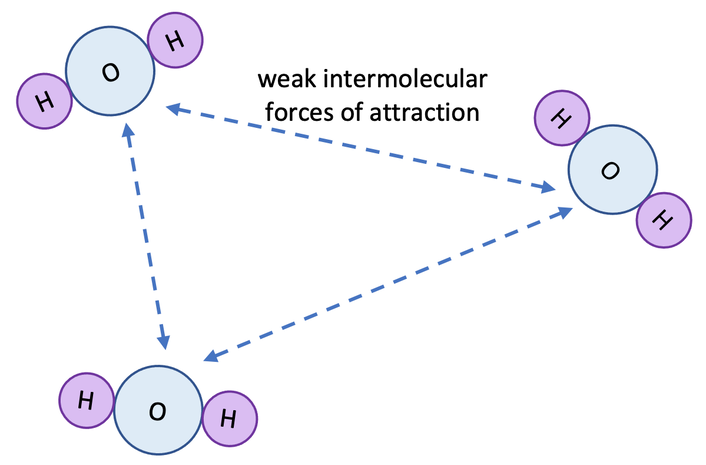
- In gases, and to a lesser extent in liquids, the intermolecular forces are weak.
- Because particles are not strongly bonded, vibrations or energy cannot easily be passed along the structure.
3. Irregular Movement:

- Particles in gases and liquids move around randomly and freely (unlike in solids where particles vibrate in fixed positions).
- This randomness makes direct energy transfer less efficient.
4. No Free Electrons:
- Unlike metals, gases and liquids do not contain free (delocalised) electrons.
- This means they lack the fast-moving carriers that help conduct thermal energy efficiently in metals.
Example:
A student places a metal spoon and a wooden spoon into a cup of hot soup. After 2 minutes, the metal spoon feels hot to the touch near the handle, while the wooden spoon still feels cool. Later, the student notices that even though steam is rising from the soup, the air above the cup does not feel very hot.
Explain all the observations using particle theory and thermal conduction concepts.
▶️ Answer/Explanation
Metal Spoon:
- Metals contain free (delocalised) electrons.
- When the spoon is placed in hot soup, thermal energy is transferred quickly through the metal by both lattice vibrations and fast-moving electrons.
- This makes the handle of the metal spoon hot quickly — metal is a good thermal conductor.
Wooden Spoon:
- Wood is a non-metal and does not have free electrons.
- Heat transfers only through slow lattice vibrations, and particles in wood are tightly held but poor at passing energy.
- So the handle of the wooden spoon remains cool — wood is a thermal insulator.
Air Above the Soup (Gas):
- Although hot water is turning into steam, the air above the soup does not feel hot.
- This is because gases have widely spaced particles, so energy is transferred poorly by collisions.
- There are no lattice vibrations or free electrons in air, so conduction is very poor — gases are very poor thermal conductors.
Final Answer: The metal spoon conducts heat quickly due to free electrons and vibrations, the wooden spoon does not because it lacks both, and the air remains cool because particles are too far apart to transfer heat efficiently.
Thermal Conductivity: A Spectrum
Thermal Conductivity: A Spectrum
Solids can be divided into three broad groups based on how well they conduct thermal energy:
| Type of Solid | Thermal Conductivity | Examples |
|---|---|---|
| Good Conductors | Excellent | Metals (copper, aluminium, silver) |
| Moderate Conductors | Medium | Glass, ceramic tiles, concrete |
| Poor Conductors (Insulators) | Very poor | Wood, plastic, rubber, wool |
Many solids-like glass or stone-conduct heat better than thermal insulators like plastic or wood, but they do not conduct as well as metals.
- They have tightly packed particles, which allow lattice vibrations to transfer energy.
- But they lack free electrons, so energy transfer is slower than in metals.
Example:
A student places three rods-one made of aluminium, one of glass, and one of wood-into a beaker of hot water. After 2 minutes, the student touches the ends of each rod furthest from the water. The aluminium rod feels very hot, the glass rod feels warm, and the wooden rod feels cool.
Explain these observations using the concept of thermal conduction in solids.
▶️ Answer/Explanation
Aluminium (metal):
- Aluminium is a good thermal conductor.
- It contains free (delocalised) electrons that transfer energy quickly through the metal.
- So heat travels rapidly from the hot water to the other end of the rod, making it feel very hot.
Glass:
- Glass is a moderate thermal conductor.
- It has tightly packed particles that can transfer heat through lattice vibrations.
- However, it has no free electrons, so conduction is slower than in aluminium.
- This is why the glass rod feels warm, but not as hot as the aluminium one.
Wood:
- Wood is a poor thermal conductor (a thermal insulator).
- It has a less regular structure and often contains trapped air, which slows heat transfer.
- Only very slow lattice vibrations occur, so the end of the rod stays cool.
Aluminium conducts heat the fastest due to free electrons, glass transfers some heat by lattice vibrations only, and wood barely conducts heat at all, making it feel cool.
