Solids, liquids and gases - CIE iGCSE Chemistry Notes - New Syllabus
Solids, liquids and gases for iGCSE
Core Syllabus
- State the distinguishing properties of solids, liquids and gases
- Describe the structures of solids, liquids and gases in terms of particle separation, arrangement and motion
- Describe changes of state in terms of melting, boiling, evaporating, freezing and condensing
- Describe the effects of temperature and pressure on the volume of a gas
Supplement Syllabus
- Explain changes of state in terms of kinetic particle theory, including the interpretation of heating and cooling curves
- Explain, in terms of kinetic particle theory, the effects of temperature and pressure on the volume of a gas
Solids, Liquids and Gases
Solids, Liquids and Gases
Solids, liquids and gases are the three main states of matter. Each state has different physical properties because of the way particles are arranged and how they move.
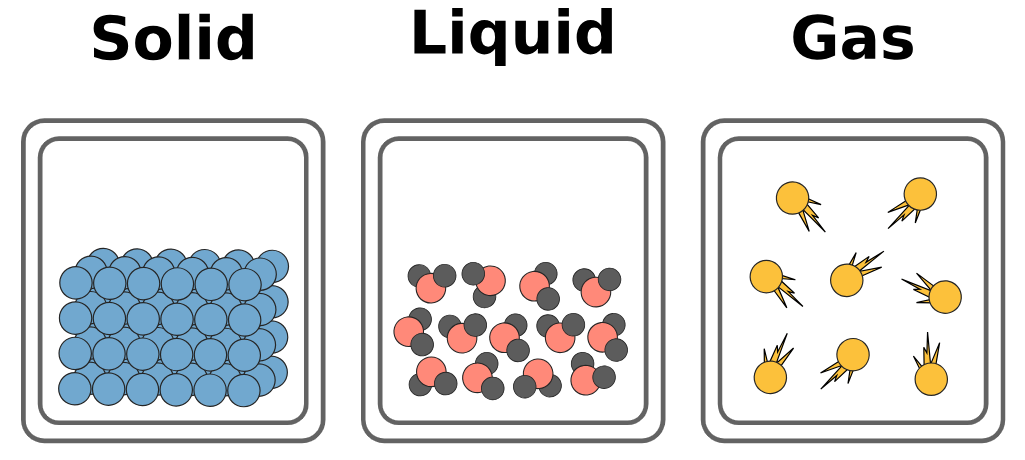
Distinguishing Properties:
| Property | Solids | Liquids | Gases |
|---|---|---|---|
| Shape | Fixed shape | No fixed shape – takes the shape of container | No fixed shape – spreads to fill container |
| Volume | Fixed volume | Fixed volume | No fixed volume – easily compressed |
| Compressibility | Not compressible | Very slightly compressible | Highly compressible |
| Density | Usually high | Moderate | Low |
| Flow | Cannot flow | Can flow | Can flow |
| Diffusion | Very slow/negligible | Occurs slowly | Occurs quickly |
Example
Compare the properties of a solid and a gas in terms of shape, volume and compressibility.
▶️Answer/Explanation
A solid has a fixed shape and volume and cannot be compressed because its particles are tightly packed in a regular structure. A gas has no fixed shape or volume and can be compressed easily because its particles are far apart and move freely.
Real-World Applications of the Properties of the Three States:
- Solids:
- Used in construction (bricks, metal bars) due to their fixed shape and strength.
- Cannot flow or be compressed – suitable for strong, stable structures.
- Liquids:
- Used in transportation of chemicals, fuels, and water – they flow and take the shape of containers.
- Incompressibility makes them useful in hydraulic systems (e.g. car brakes).
- Gases:
- Used in inflating tyres, balloons, and airbags – compressible and expand to fill space.
- Essential in breathing systems – oxygen tanks and gas cylinders.
Example
State two reasons why a liquid can be poured while a solid cannot.
▶️Answer/Explanation
In a liquid, particles are close but can slide past each other, allowing the liquid to flow. In a solid, particles are fixed in position and can only vibrate, so solids do not flow or take the shape of a container.
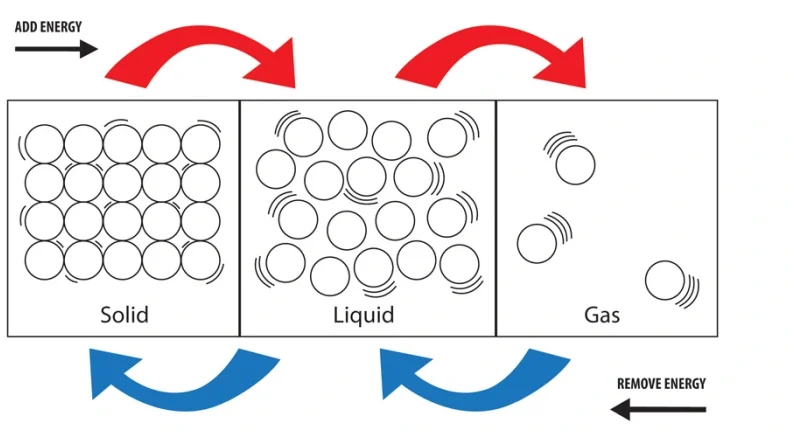
Structures of solids, liquids and gases in terms of particle separation, arrangement and motion
The physical properties of solids, liquids and gases can be explained by looking at how their particles are arranged, how close they are to each other, and how they move. This is the basis of the kinetic particle theory.
Structure of Solids:
- Separation: Particles are tightly packed together with very little space between them.
- Arrangement: Particles are arranged in a fixed, regular pattern (a lattice).
- Motion: Particles vibrate in fixed positions but do not move from place to place.
- Result: Solids have a fixed shape and volume. They are rigid and not compressible.
Structure of Liquids:
- Separation: Particles are close together but with small spaces between them.
- Arrangement: Particles are not in a fixed pattern – they are randomly arranged.
- Motion: Particles can move and slide past each other.
- Result: Liquids have a fixed volume but no fixed shape. They can flow and are slightly compressible.

Structure of Gases:
- Separation: Particles are far apart with large gaps between them.
- Arrangement: Particles are randomly arranged and spread out.
- Motion: Particles move freely and rapidly in all directions.
- Result: Gases have no fixed shape or volume. They can be compressed and expand to fill their container.
| State | Particle Separation | Particle Arrangement | Particle Motion |
|---|---|---|---|
| Solid | Very close together | Regular, fixed pattern | Vibrate only |
| Liquid | Close together | Random, not fixed | Move around and slide |
| Gas | Very far apart | Random, widely spaced | Rapid, random motion |
Changes of state in terms of melting, boiling, evaporating, freezing and condensing
Changes of state in terms of melting, boiling, evaporating, freezing and condensing
Changes of state are physical changes where a substance changes from one state of matter to another – solid, liquid or gas – without changing its chemical identity.
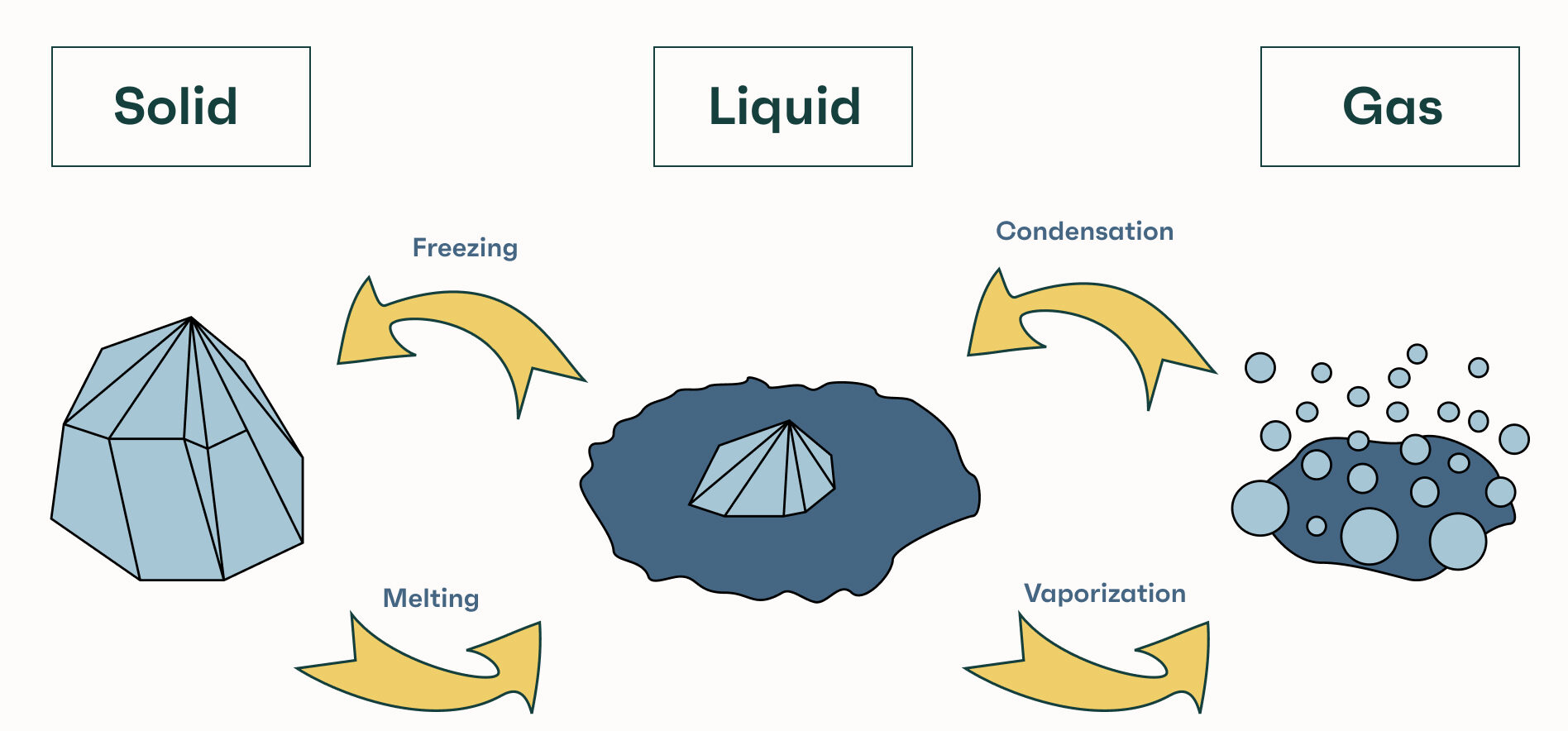
The Main Changes of State:
- Melting: Solid → Liquid
- Boiling: Liquid → Gas (throughout the liquid at boiling point)
- Evaporation: Liquid → Gas (at the surface, below boiling point)
- Freezing: Liquid → Solid
- Condensation: Gas → Liquid
Descriptions of Each Process:
Melting:
- When a solid is heated, its particles gain kinetic energy and vibrate more strongly.
- At the melting point, vibrations become strong enough to overcome the forces holding particles in fixed positions.
- The solid becomes a liquid – particles are still close but can move around.
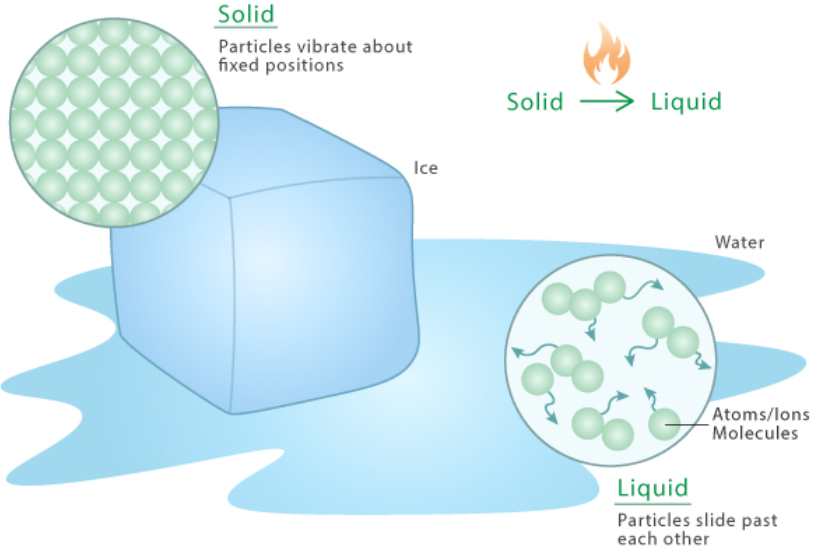
Boiling:
- As a liquid is heated, its particles gain more kinetic energy.
- At the boiling point, particles throughout the liquid have enough energy to overcome intermolecular forces and escape as gas.
- Bubbles of gas form within the liquid and rise to the surface.

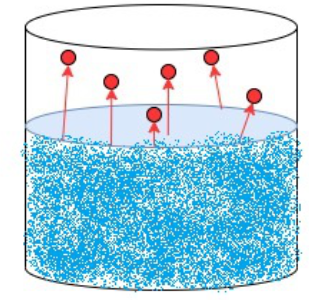
Evaporation:
- Evaporation occurs at any temperature (not just boiling point), but only at the surface of the liquid.
- Some particles at the surface have enough kinetic energy to escape into the air as gas.
- It is a slower process than boiling and causes cooling (particles with higher energy escape, leaving behind lower-energy particles).
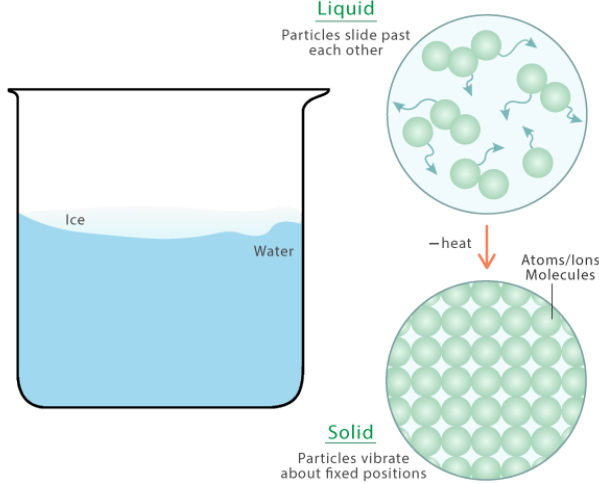
Freezing:
- When a liquid is cooled, its particles lose kinetic energy and move more slowly.
- At the freezing point, particles no longer have enough energy to overcome the attractive forces between them.
- They settle into fixed positions, forming a solid.
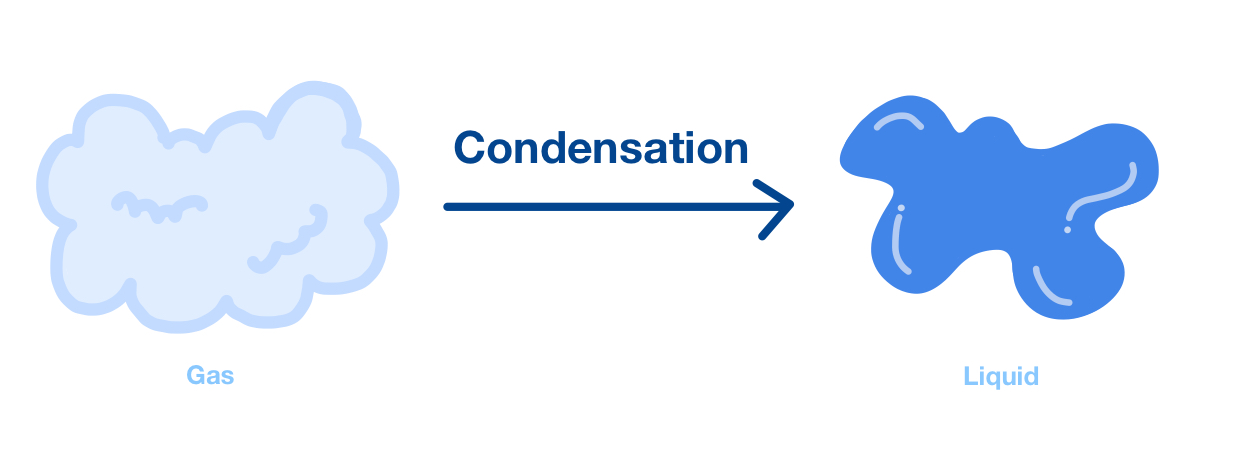
Condensation:
- When a gas is cooled, its particles lose kinetic energy and move more slowly.
- Eventually, particles come close enough for attractive forces to pull them together into a liquid.
Key Ideas to Remember:
- These are all physical changes – the substance remains the same but in a different form.
- No new substances are formed.
- Changes of state involve changes in energy (usually heat).
| Change | From | To | Energy Change | Type of Process |
|---|---|---|---|---|
| Melting | Solid | Liquid | Gain heat | Endothermic |
| Boiling | Liquid | Gas | Gain heat | Endothermic |
| Evaporation | Liquid (surface) | Gas | Gain heat | Endothermic |
| Freezing | Liquid | Solid | Lose heat | Exothermic |
| Condensation | Gas | Liquid | Lose heat | Exothermic |
Example
Explain what happens to the particles in a solid when it melts to form a liquid.
▶️Answer/Explanation
When the solid is heated, particles gain energy and vibrate more strongly. At the melting point, the particles have enough energy to break free from their fixed positions. They begin to move past each other but stay close together, forming a liquid.
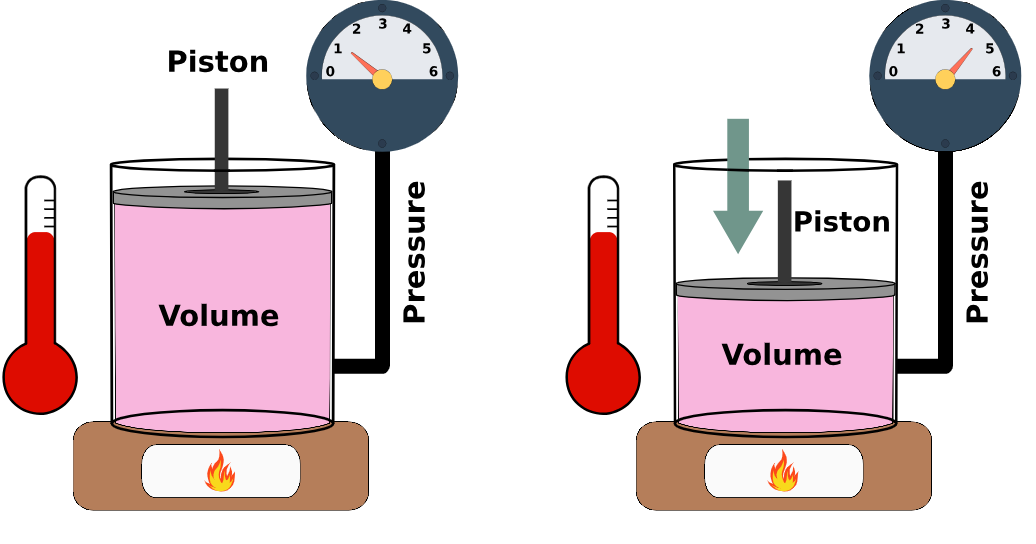
Effects of temperature and pressure on the volume of a gas and Kinetic Particle Theory
Effects of temperature and pressure on the volume of a gas
The volume of a gas is affected by changes in temperature and pressure. These relationships can be explained using the kinetic particle theory.
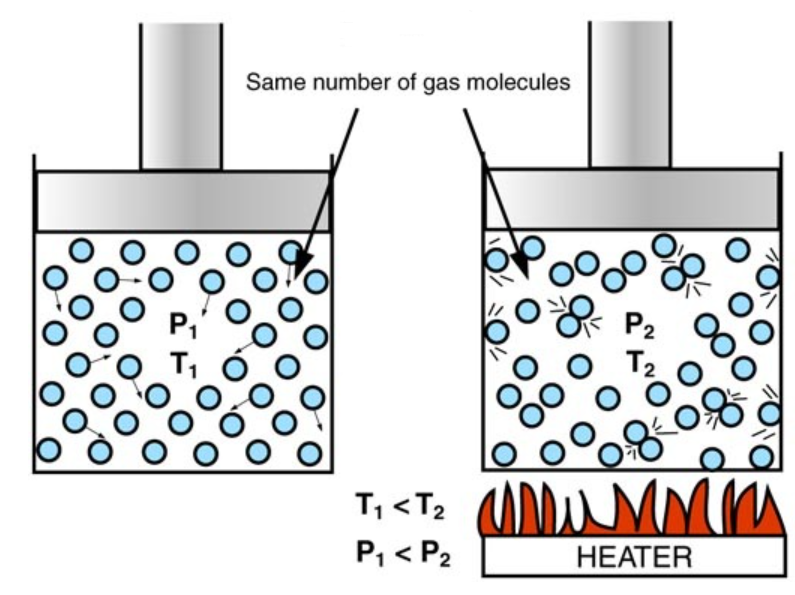
Effect of Temperature on Gas Volume:
- As temperature increases, gas particles gain kinetic energy and move faster.
- They hit the walls of the container more often and with more force.
- If the pressure is kept constant, the gas expands – the particles need more space to move.
- Conclusion: At constant pressure, increasing temperature increases gas volume. This is known as Charles’s Law.
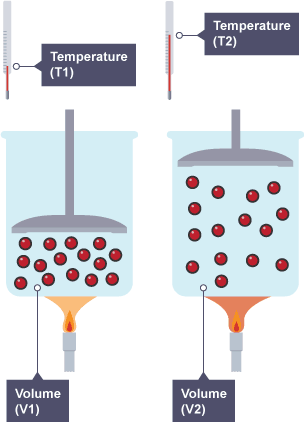
Mathematically:
If pressure is constant, \( \frac{V}{T} = \text{constant} \)
(where \( V \) = volume, \( T \) = temperature in Kelvin)
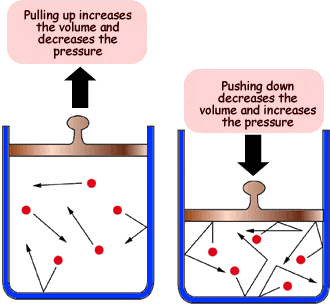
Effect of Pressure on Gas Volume:
- When pressure is increased, the gas particles are forced closer together.
- This decreases the volume because the particles now occupy a smaller space.
- If temperature remains constant, gas particles continue moving at the same speed, but collide more often with the walls.
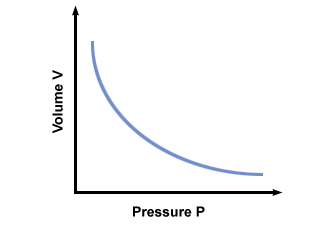
- Conclusion: At constant temperature, increasing pressure decreases volume. This is known as Boyle’s Law.
Mathematically:
If temperature is constant, \( P \times V = \text{constant} \)
(where \( P \) = pressure, \( V \) = volume)
Gas Relationships:
- Charles’s Law:
At constant pressure → Temperature ↑ → Volume ↑
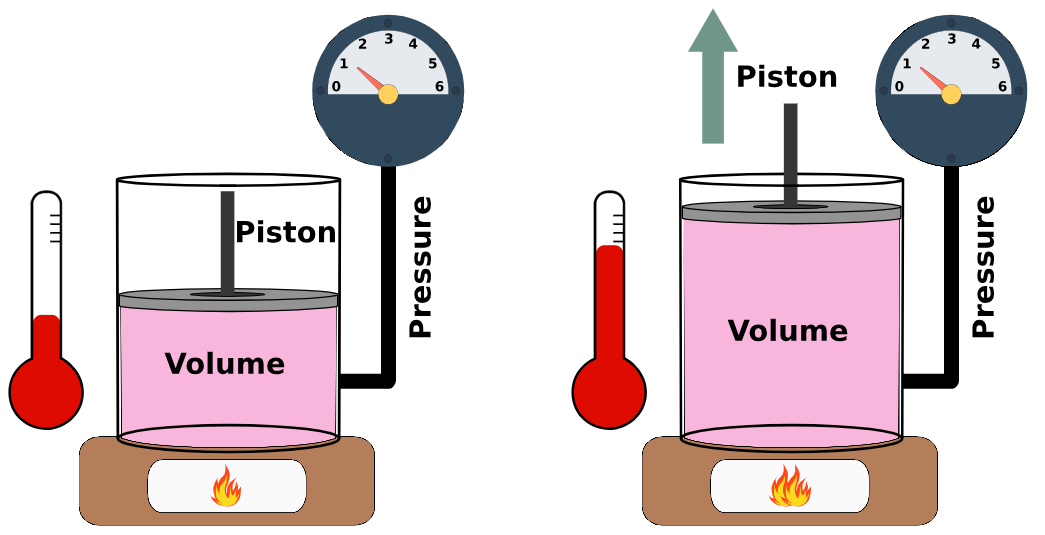
- Boyle’s Law:
At constant temperature → Pressure ↑ → Volume ↓
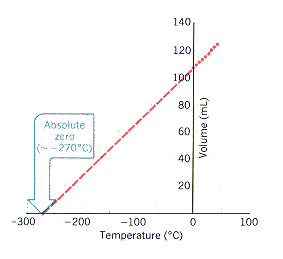
Graphical Representations:
- Charles’s Law: Straight line graph of \( V \) vs \( T \) (in Kelvin)
- Boyle’s Law: Curve for \( P \) vs \( V \); straight line for \( P \) vs \( \frac{1}{V} \)
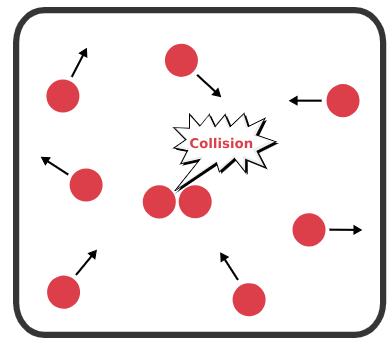
Kinetic Particle Theory and Gas Behavior:
Gases consist of particles that are far apart and move randomly in straight lines. The behavior of gases is explained by how these particles gain or lose energy when temperature or pressure changes.
- Gas particles are always in motion and collide with container walls – this causes pressure.
- When temperature increases, particles move faster → more collisions → volume increases (if pressure is fixed).
- When gas is compressed, particles have less space → more collisions → pressure increases (if temperature is fixed).
Example
Explain, using kinetic particle theory, what happens to the volume of a gas when it is cooled while pressure remains constant.
▶️Answer/Explanation
When a gas is cooled, its particles lose kinetic energy and move more slowly. They hit the walls of the container less frequently and with less force. To maintain constant pressure, the gas contracts and its volume decreases.
Changes of state in terms of kinetic particle theory, and the interpretation of heating and cooling curves
Changes of state in terms of kinetic particle theory, and the interpretation of heating and cooling curves
Kinetic particle theory explains how particles behave during heating and cooling. Changes of state occur when a substance gains or loses energy, causing its particles to move differently.
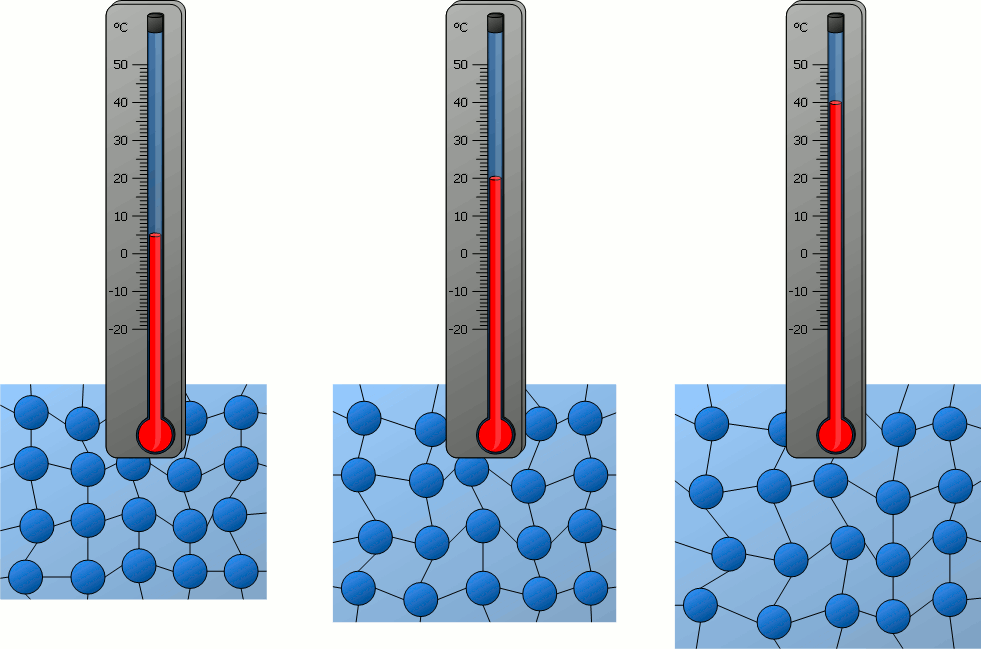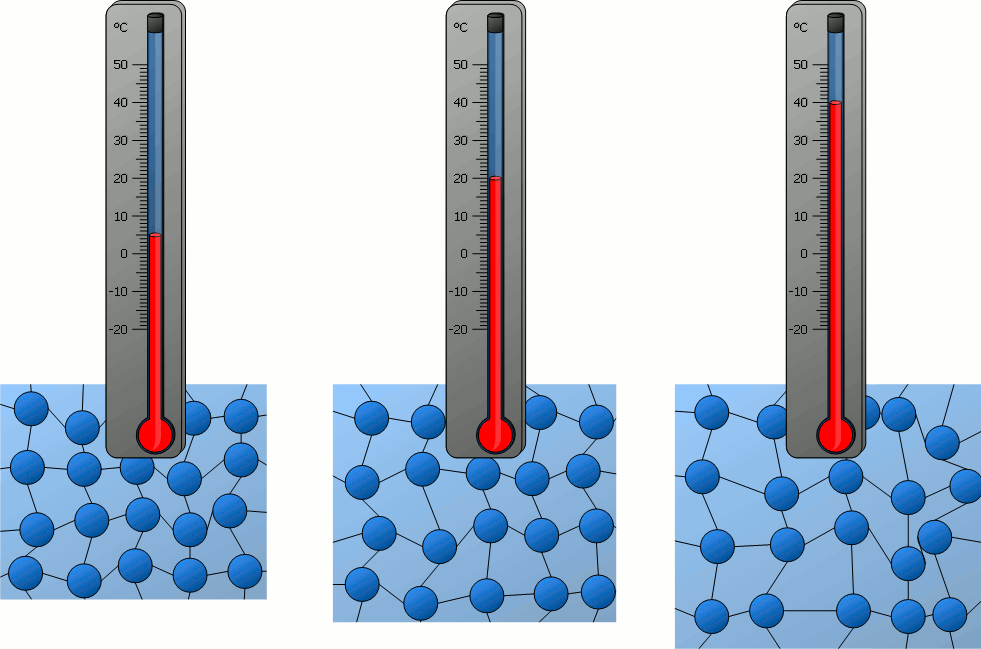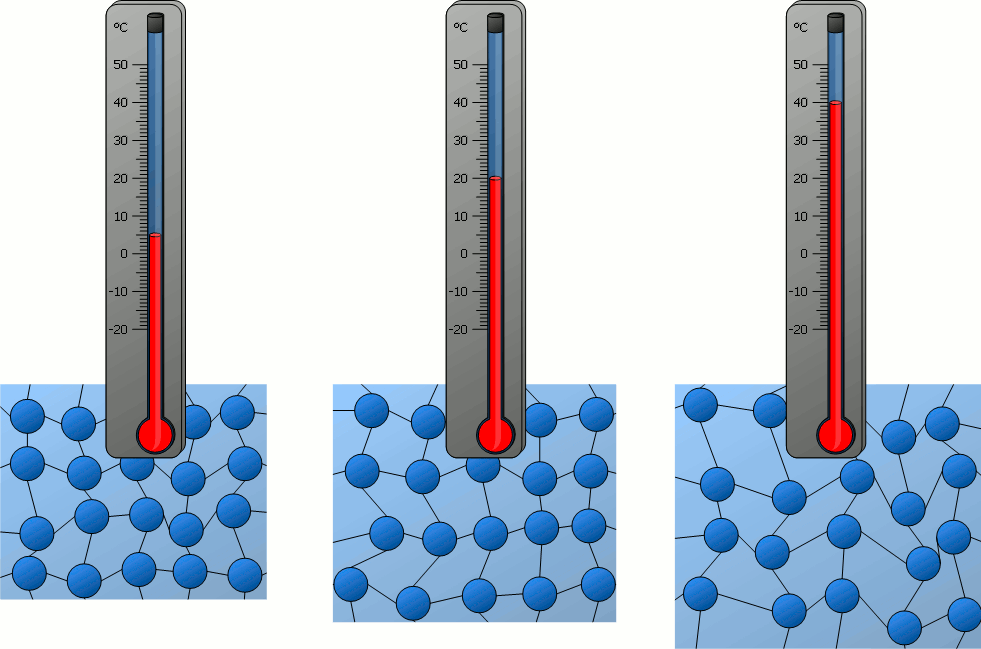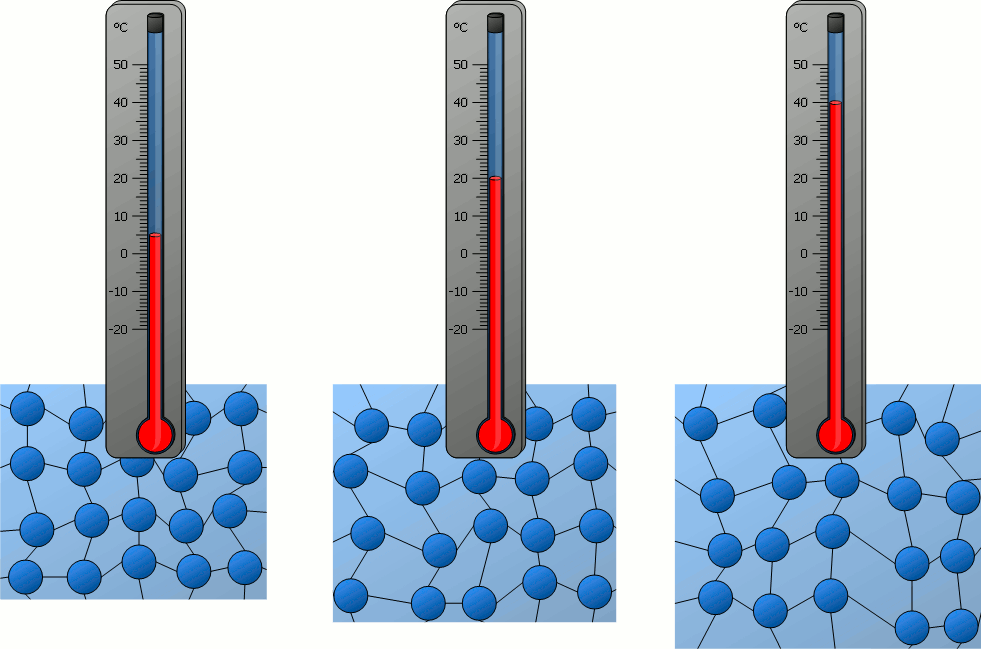
During Heating:
- When a substance is heated, its particles absorb energy.
- This energy increases their kinetic energy, making them move faster.
- If enough energy is gained, particles overcome the attractive forces holding them together, leading to a change of state.
During Cooling:
- As a substance cools, its particles lose energy and move more slowly.
- Eventually, the particles no longer have enough energy to remain in their current state.
- This leads to changes like condensation (gas → liquid) or freezing (liquid → solid).
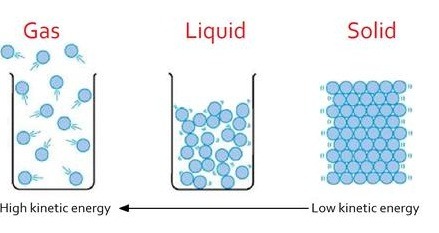
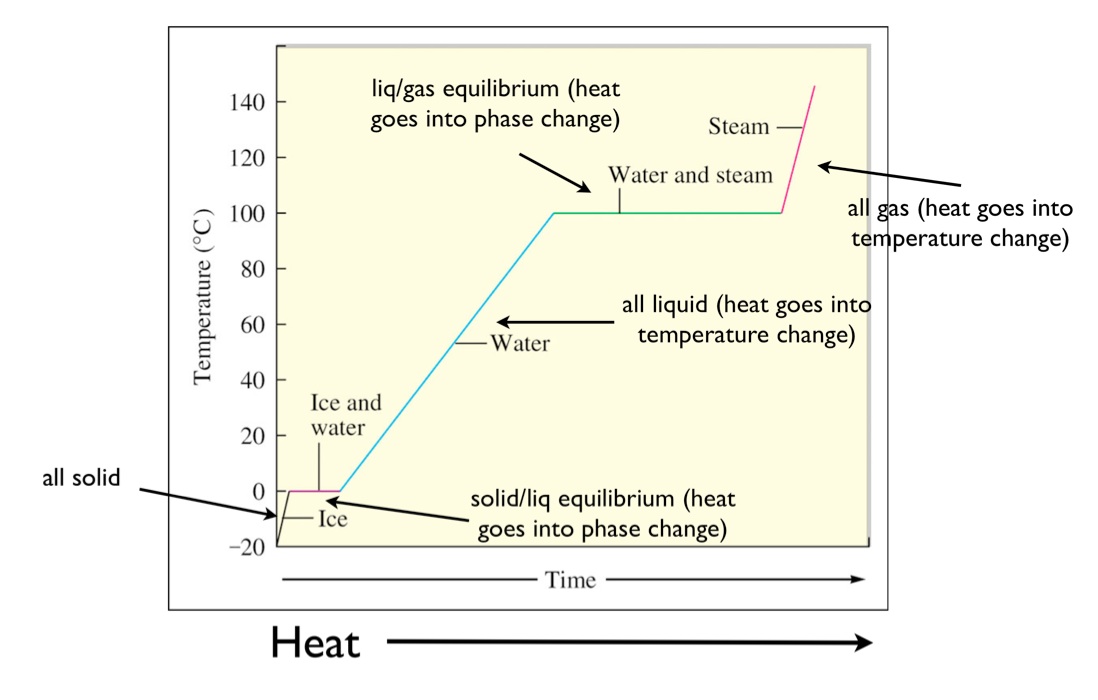
Heating Curve:
A heating curve shows how the temperature of a substance changes as it is heated at a constant rate.
- Solid heating up: Temperature rises as particles vibrate more (kinetic energy increases).
- Melting point: Flat section – temperature stays constant while particles gain enough energy to overcome forces and change to liquid. Energy is used to break bonds, not increase temperature.
- Liquid heating up: Temperature rises again – particles move faster and spread out.
- Boiling point: Another flat section – temperature remains constant while particles break free from liquid and become gas.
- Gas heating up: Temperature rises again as particles move very quickly and spread far apart.
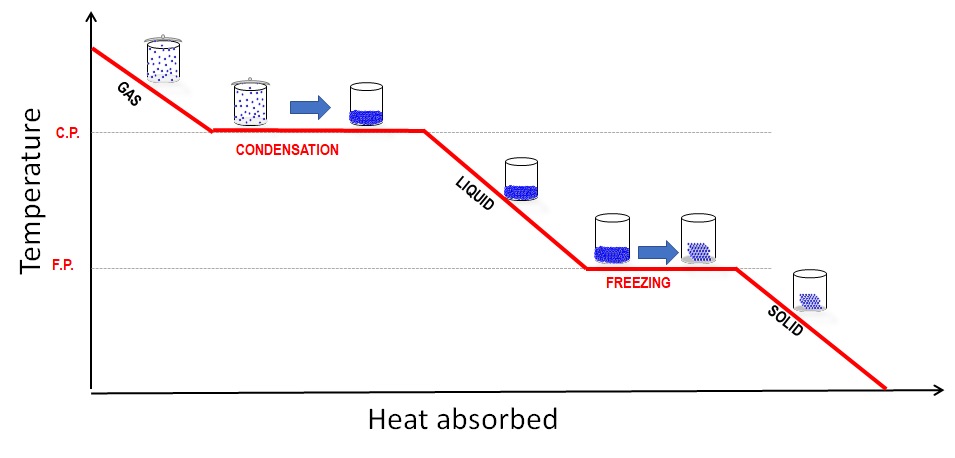
Cooling Curve:
A cooling curve is the reverse process. As energy is lost, particles slow down, and the substance goes through condensation and freezing.
- Gas cooling: Temperature drops as particles lose kinetic energy.
- Condensation: Temperature stays constant while gas changes to liquid – energy is released as bonds form.
- Liquid cooling: Particles move slower as more heat is lost.
- Freezing: Temperature remains constant while liquid becomes solid – energy is released.
Why Temperature Stays Constant During State Change:
- During melting or boiling, energy added is used to break forces between particles (overcoming attractive forces).
- During freezing or condensing, energy is released as particles come closer and form bonds.
- No temperature change occurs during these phases even though energy is still being transferred.
Key Points to Remember:
- Temperature rises only when kinetic energy increases.
- Flat sections of the graph mean a state change is occurring.
- Kinetic particle theory explains why energy is needed to change state even when the temperature doesn’t change.
Example
Explain why the temperature remains constant during the melting of a solid, even though heat is still being added.
▶️Answer/Explanation
During melting, the energy supplied is not used to increase kinetic energy, but to overcome the forces between the particles. This energy allows the particles to break out of their fixed positions in the solid and move freely as a liquid. Since this energy goes into breaking forces rather than increasing speed, the temperature stays the same.
