Question (10 marks)
These animations show the two different types of wave moving along springs.
Question a (2 mark)
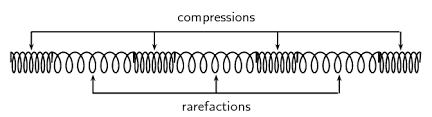
Label the two diagrams below to show wavelength, amplitude, compression and rarefaction.
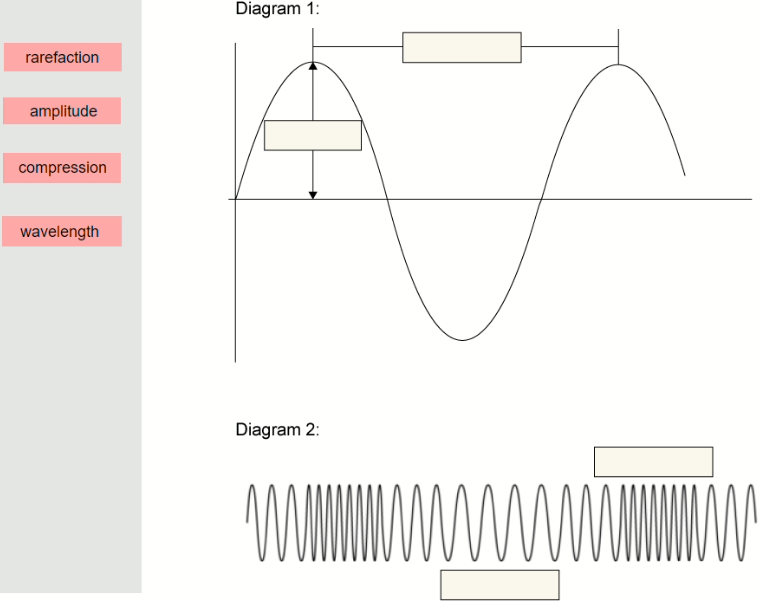
▶️Answer/Explanation
Ans:

Question b (1 mark)
Sound waves can be represented on a computer. Below are some waves recorded on a computer from four different sources.
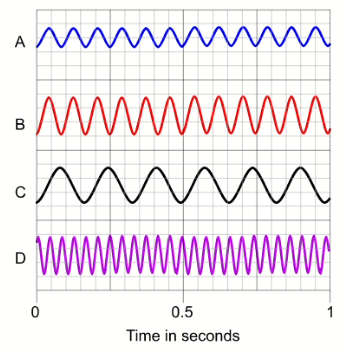
Calculate the frequency of wave $\rm A$.
▶️Answer/Explanation
Ans:
Question 2c (1 mark)
Identify which two waves have the same frequency.
▶️Answer/Explanation
Ans:
Question d (2 mark)
Calculate the wavelength of wave C.
▶️Answer/Explanation
Ans:
Question e (1 mark)
Infrasound waves have a frequency of less than $20 \mathrm{~Hz}$. Use your answers above to identify which wave does not show infrasound.
▶️Answer/Explanation
Ans:
Understanding and using standard form
People regularly have to communicate large or small numbers and our language has words such as million or thousandth that help us to do this. The International System of Units, referred to as the SI system, also has prefixes which help communicate large or small units. For example, a kilometer is one thousand meters and a microgram is a millionth of a gram.
Some other prefixes used with SI units are shown below.

Scientists often need to express numbers which are beyond this scale. The mass of an electron is 0.91 thousandths of a yoctogram (the prefix yocto means 10–24 and is so small that it is rarely used) and so you would need about one million million million million million electrons to make a kilogram. Neither of these numbers is easy to communicate. Standard form makes it easier to represent large or small numbers. In standard form, we would write that the mass of an electron is 9.1 × 10–31 kg and so you would need just over 1 × 1030 electrons to make a kilogram.
Question:
Express these numbers in standard form:
a) The probability of shuffing a pack of cards and finding that they had ended up in sequential order is one in eighty million million million million million million million million million million million.
▶️Answer/Explanation
Ans: 8 × 1067
b) The number of insects on the Earth is estimated to be ten million million million.
▶️Answer/Explanation
Ans: 1 × 1019
c) The number of protons in the universe is thought to be about one hundred million million million million million million million million million million million million million.
▶️Answer/Explanation
Ans: 1 × 1080
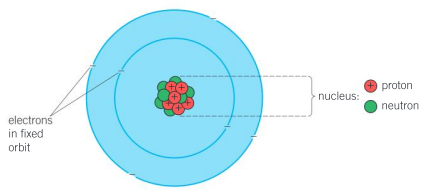
In Rutherford’s model of the atom, the nucleus consists of protons and neutrons, and the electrons are in fixed orbits around the nucleus. The overall atom has no charge, since there are the same number of electrons as protons
Question:
Why do you think that the electron was the easiest of these three particles to discover?
▶️Answer/Explanation
Ans: Electrons surround the nucleus. They are on the outside of the atom and so interact more easily with the outside world.
Question:
Why do you think the neutron might have been the hardest of these particles to discover?
▶️Answer/Explanation
Ans: Neutrons have no charge and are part of the nucleus.
Question:
Any given atom will have the same number of electrons as protons. For light elements it is likely to have the same number of neutrons as protons. For example, an atom of nitrogen taken from the air has seven protons and seven neutrons in its nucleus, and there are seven electrons which orbit around the nucleus. What proportion of the particles in the atom are electrons? What fraction of the mass is in the electrons?
▶️Answer/Explanation
Ans: In a nitrogen atom, \(\frac{7}{21}=\frac{1}{3}\) particles are electrons. Fraction of mass in electrons = \(\frac{7\times 0.00055}{7\times 0.00055+14\times 1}\)
\(=2.75\times 10^{-4}\)
Question:
Here are some atoms written in atomic notation: \(_{7}^{14}\textrm{N},_{8}^{14}\textrm{O},_{6}^{13}\textrm{C},_{6}^{14}\textrm{C}.\)
a) Which atom has more protons than neutrons?
▶️Answer/Explanation
Ans: \(_{8}^{14}\textrm{O}\)
b) Which atom has the most neutrons?
▶️Answer/Explanation
Ans: \(_{6}^{14}\textrm{C}\)
c) Which two atoms are isotopes of each other?
▶️Answer/Explanation
Ans: \(_{6}^{13}\textrm{C} and _{6}^{14}\textrm{C}\)
d) In a radioactive process, \(_{6}^{14}\textrm{C}\) changes one of the neutrons in its nucleus into a proton. Which atom has it turned into?
▶️Answer/Explanation
Ans: \( _{7}^{14}\textrm{N}\)
Question:
The graph below shows the depth of water in a harbor as a wave passes through.
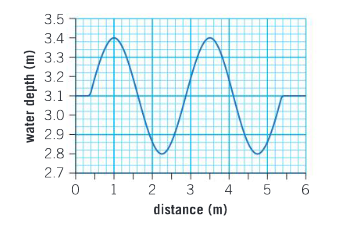
a. From the graph, measure the wavelength of the wave.
▶️Answer/Explanation
Ans: Measuring peak to peak gives 3.5 – 1 = 2.5 m
b. Determine the amplitude of the wave.
▶️Answer/Explanation
Ans: \(\frac{3.4-2.8}{2}=0.3m\)
c. The speed of the waves is 1.4 m s–1. Calculate how long it takes a wave to pass a given point.
▶️Answer/Explanation
Ans: \(f=\frac{1.4}{2.5}=0.56 Hz; T = \frac{1}{0.56}=1.79s\)
Summative assessment
Probing the atom
As a general rule, waves can only be used to see objects that are larger than the wavelength of the waves. Since the wavelength of visible light is about a thousand times larger than an atom, an optical microscope cannot be used to see individual atoms. The nuclei of atoms are much smaller still and so we require waves with very small wavelengths to probe the nucleus of atoms. Electrons demonstrate both a wave-like and a particle-like behavior and since the wavelength of high energy electrons can be very small, they can be used to probe the nuclei of atoms. In an experiment to measure the size of the nucleus of a gold atom, the wavelength of the electrons is 2 × 10–16 m and they are traveling at 3 × 108 m s–1.
Question:
Calculate the frequency of the electron wave.
▶️Answer/Explanation
Ans: \(\frac{3\times 10^{8}}{2\times 10^{-16}}=1.5\times 10^{24}Hz\)
Question:
Calculate the time period of the electron waves.
▶️Answer/Explanation
Ans: \(\frac{1}{1.5\times 10^{24}}=6.7\times 10^{-25}s\)
Question:
The target nucleus in the experiment was gold which has a mass number of 197 and an atomic number of 79.
a) Describe this nucleus in atomic notation. (The chemical symbol for gold is Au.)
▶️Answer/Explanation
Ans: \(_{79}^{197}\textrm{Au}\)
b) How many neutrons are in the gold nucleus?
▶️Answer/Explanation
Ans: 197 – 79 = 118
Question:
Another isotope of gold has a mass number of 200. Explain what is meant by an isotope and how these nuclei differ from the gold197 nuclei.
▶️Answer/Explanation
Ans: Atoms of the same element, which have the same number of protons; but different numbers of neutrons; gold-200 nuclei contain 3 more neutrons than gold-197 nuclei.
Question:
Explain why the two gold isotopes have similar chemical properties.
▶️Answer/Explanation
Ans: Both isotopes have the same number of protons so same configuration of electrons; electrons are responsible for the chemical properties of an atom; The chemical properties of each isotope are therefore similar.
Question:
The electron waves are transverse. Describe the difference between a transverse wave and a longitudinal wave.
▶️Answer/Explanation
Ans: In transverse waves, matter moves at right angles (perpendicular) to the direction in which the wave is traveling; In longitudinal waves, matter moves parallel to the direction in which the wave is travelling
Investigating the nuclear radius
A series of experiments is designed to investigate other nuclear radii.
Question:
Explain which of the following you think would be the most suitable independent variable for the experiment:
atomic number mass number number of electrons.
▶️Answer/Explanation
Ans: Number of electrons is unsuitable because the electrons are not in the nucleus; changing the atomic number would only change the number of protons –nuclear radius could still change on account of the number of neutrons; massnumber is most suitable.
Also accept that changing mass number means that nuclear charge can be kept constant as a control by using isotopes of the same element (i.e. keeping the number of protons fixed).
Question:
Write a suitable hypothesis for this experiment.
▶️Answer/Explanation
Ans: Award marks for: suitable hypothesis; links independent and dependent variables; based on reasoning. For example, as the mass number is increased, the radius of the nucleus will increase because each additional nucleon increases the volume of the nucleus (may use \(V = \frac{4}{3}\pi r^{3}\) for spherical nucleus).
Question:
One suggestion is to investigate and measure the different radii of the isotopes of gold. Discuss whether this is a good suggestion
▶️Answer/Explanation
Ans: Using only one isotope means that the number of protons in the nucleus is always the same; charge is a controlled variable; limited number of isotopes (gold only has one stable isotope); some isotopes will have short half-lives; limited range of mass number; not much variation in radius between isotopes.
Question:
Explain why it might be important to use the same wavelength of electrons when measuring the differing nuclei.
▶️Answer/Explanation
Ans: Using different wavelengths might give different results; electron wavelength is a controlled variable; allows comparison between different measurements.
The liquid drop model of the nucleus
Question:
The graph below shows the nuclear radius of some nuclei in femtometers (1 fm = 1 × 10–15 m).
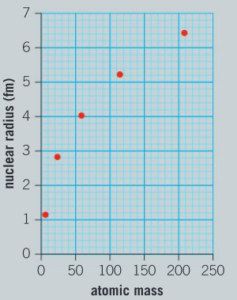
a) Would you classify the trend of the graph as directly proportional, linear or non-linear?
▶️Answer/Explanation
Ans: Non-linear
b) Draw a line of best fit on a copy of the graph.
▶️Answer/Explanation
Ans: Curved line through points.
c) Use the graph to predict the radius of a nucleus of tungsten-184.
▶️Answer/Explanation
Ans: 6.2 fm (allow ±0.1 fm)
Question:
A model of the nucleus called the liquid drop model suggests that the volume of a nucleus is directly proportional to the number of protons and neutrons in it.
A graph of the volume of nuclei against mass number is shown below.
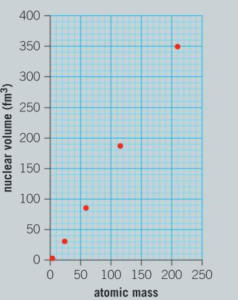
a) Using your value of the radius of tungsten-184 from the first graph, calculate the volume of this nucleus. (Assume that the nucleus is a sphere.)
Answer/Explanation
▶️Answer/Explanation
Ans: \(V = \frac{4}{3}\pi r^{3}=\frac{4}{3}\pi (6.2)^{3}=998 fm^{32}\)
b) How would you classify the trend of this graph?
Answer/Explanation
▶️Answer/Explanation
Ans: Directly proportional.
c) Add this data point to a copy of the graph.
Answer/Explanation
▶️Answer/Explanation
Ans: The values on the y-axis of the graph in the first edition of the MYP Physics book are incorrect. The correct values are given in the graph below, along with the data point for tungsten-184.
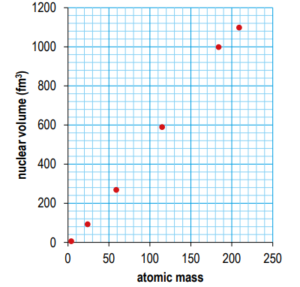
d) Discuss whether the liquid drop model of the nucleus appears to be a good model. You should refer to the graph in your answer.
Answer/Explanation
▶️Answer/Explanation
Ans: Arguments for it being a good model: model predicted a directly proportional relationship and the experimental data agrees; additional point fits the trend; model can be used to make predictions; model is a simple explanation.
Arguments against it being a good model: data points are not exactly on straight line; model does not explain the discrepancy. To score 4 or 5 marks, answer should include at least one point for it being a good model and against.
Describing the atom
Question:
The experiment described in this section can be described as nuclear physics since it is the study of the nucleus. However, the words “nuclear” and “atomic” are sometimes thought to refer
to nuclear weapons and can cause fear as a result. Write a short paragraph explaining the structure of an atom without using the words “nuclear” or “atomic”.
▶️Answer/Explanation
Ans: Award credit for an accurate description of the structure of an atom without using the words “nuclear” and “atomic”.
Question:
Our increased knowledge of the structure of the atom and its nucleus have been a significant advance in scientific understanding. Identify the benefits and limitations that these scientific advances have brought us and justify whether this progress has been beneficial to humankind.
▶️Answer/Explanation
Ans: Award credit for well-reasoned arguments with clear examples cited. A good answer should include benefits as well as limitations and draw a conclusion. Benefits could include the use of radioactive isotopes in industry and/or medicine and scientific progress as a beneficial increase in knowledge. Limitations might include nuclear warfare and dangers of radioactive isotopes.
