Topic 2: Molecular Biology
2.2 Water
The structure of water molecules
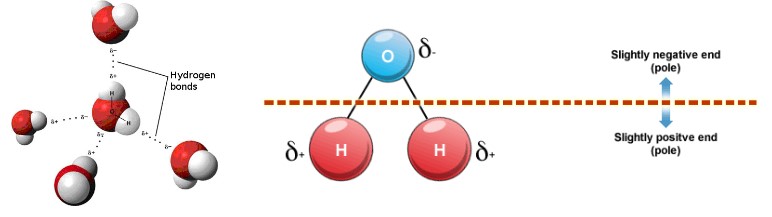
- A water molecule consists of an oxygen atom covalently bound to two hydrogen atoms
- Since O is more electronegative than H, an unequal sharing of electrons occurs
- This creates a polar covalent bond, with H having a partial positive charge and O having a partial negative charge
- Water is also bent so the positive charge exists more or less on one side and the negative charge from the O exists on the opposite side
- The partial +ve charge is attracted to the partial –ve charge creating an intermolecular attraction between the water molecules called a “Hydrogen bond.”
- H-bonds are the strongest of the intermolecular bonding, but is still considered a weak bond; however since there are so many \(H_2O\) molecules they give water its unique properties and make it essential to life on this planet
- The covalent bonds between the oxygen atom and the two hydrogen atoms of a single water molecule are categorized as polar bonds
Cohesive properties
- Water is a polar molecule, with a negative oxygen end and a positive hydrogen end.
- Hydrogen bonds that exist between water molecules create a high level of attraction linking water molecules together. This attraction
between two of the same molecules is called cohesion. - These cohesive forces allow water to move up vascular tissue in plants against gravity. It also creates surface tension on water that
allows some organisms to walk on water.

Adhesive properties
- Not only does water bind strongly to itself, it also forms H-bonds with other polar molecules. This is called adhesion.
- This is an important property in transpiration as well, as water adheres to the cellulose in the walls of the xylem vessels
- As water is evaporated from the stomata, the adhesion can help the water move up through the xylem

Thermal properties
- Water has a high specific heat capacity (amount of energy needed to raise temperature of a substance by a certain temperature level). Basically, water can absorb a lot of heat and give off a lot of heat without drastically changing the temperature of water.
- Water’s high specific heat capacity results from the extensive hydrogen bonding between the water molecules.
- Water also has a high latent heat of vaporization which means it takes a lot of heat to evaporate water from a liquid to a vapor. This is very important as a cooling mechanism for humans. As we sweat, the water droplets absorb heat from our skin causing the water to evaporate and our bodies to cool down.
- Water has thermal properties, one of which is high specific heat. This means water can absorb or give off a great deal of heat without changing temperature very much
Solvent properties
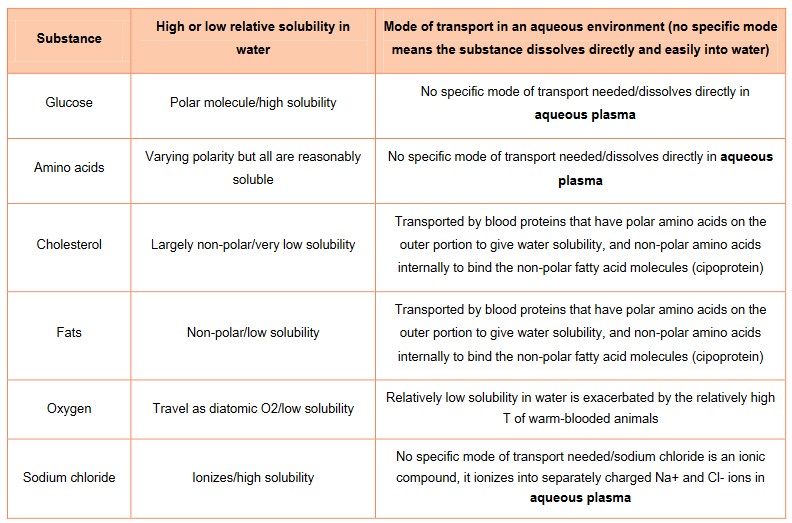
- Water is known as the “universal solvent” because of its ability to dissolve many substances because of its polarity.
- Water is able to dissolve other polar molecules such as many carbohydrates, proteins and DNA; and positively and negatively charged ions such as Na+.
- This is essential because it allows water to act as a transport medium (blood and cytoplasm) of important molecules in biological organisms.
- Water is also the medium in which most of the biochemistry of a cell occurs. A cell contains a wide variety of fluids, all of which most of the biochemistry.
Hydrophilic & hydrophobic
- Essentially hydrophilic means “water loving”
- Any substances that dissolves in water including charged ions such as Na+ or polar molecules such as glucose and fructose are
hydrophilic. Molecules that are attracted to water like phospholipid heads are also hydrophilic - Hydrophobic molecules are kind of “water fearing” but basically, these are non-polar, insoluble in water or non-charged substances, such as lipids
- Molecules such as water, that are polar substances are said to be hydrophilic, or water-loving
- Molecules that are classified as non-polar are said to be hydrophobic, or water-fearing
Comparison of the thermal properties of water with those of methane
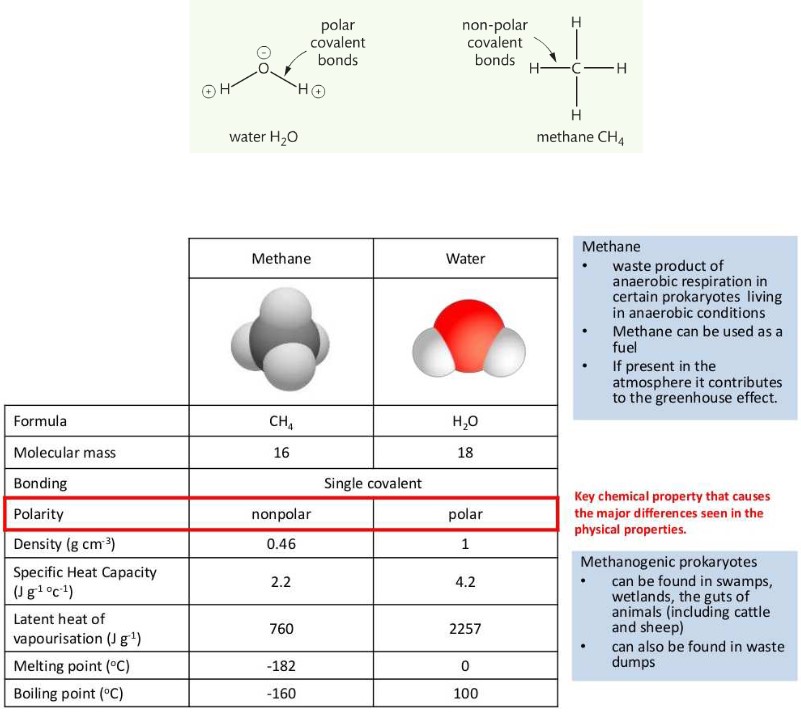
Polarity of different molecules
Water used as a coolant
- Water is essential to living organisms.
- Water has a high latent heat of vaporization which means it takes a lot of heat to evaporate water from a liquid to a vapor.
- This is very important as a cooling mechanism for living organisms. As humans sweat, the water droplets absorb heat from the blood flowing under our skin causing the water to evaporate and our blood to cool down. This will in turn cool our whole body down.
- This cooling is controlled by negative feedback through receptors in the hypothalamus
- If the body is overheated, receptors in the hypothalamus sense this and stimulate the sweat glands to secrete sweat
- Some reptiles such as crocodiles cool by opening their mouths (gaping).
