Topic 2: Molecular Biology
2.3 Carbohydrates and lipids
Monosaccharide: the building blocks of polysaccharides
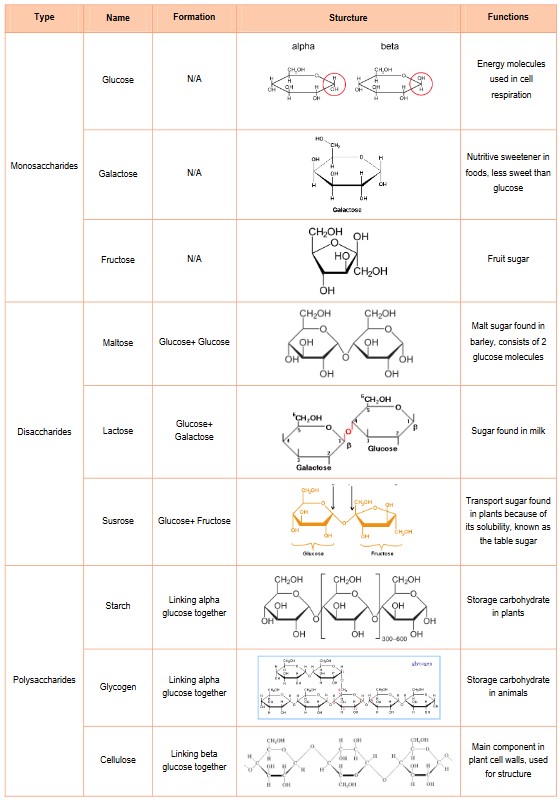
- When two monomers combine together they form a dimer. When many monomers combine together they form a polymer.
- Condensation Reactions: The building of large macromolecules (polymers) by the removal of water molecules when monomers combine. Each time two monomers combine, one water is removed.
- For example: Glucose is a monosaccharide that is used to build up large storage molecules (polysaccharides) in plants and animals. In plants, many glucose molecules combine through condensation reactions to form the polysaccharide starch. In animals, glucose molecules are combined to form the polysaccharide glycogen through condensation reactions.
- Bonds between two sugar are called glucosidic bond
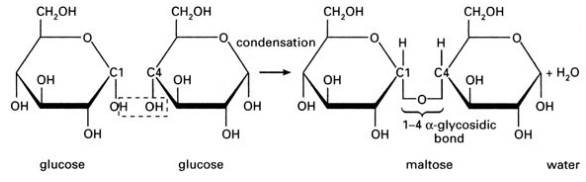
- When a plant or an animal needs to use energy stored in polysaccharide molecules, the opposite reaction to condensation takes place. This break down of larger polysaccharides into smaller monosaccharides through the addition of water is called hydrolysis (water split or separate).
- Starch and glycogen are broken down by the addition of water into glucose molecules (the energy molecule used in aerobic respiration).
- Remember: anabolism is endothermic and acatabolism is exothermic
Structure of cellulose, starch and glycogen
- Polysaccharides are long chains of monosaccharides held together with glycosidic linkages made by condensation reactions
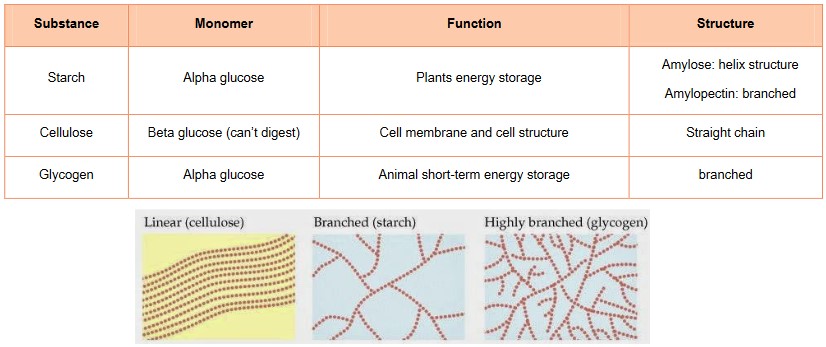
- Starch, cellulose and glycogen are all polysaccharides that are made from long chains of glucose; however, they differ in their structure
and the type of glucose, which leads to different functions - Starch consists of two types of molecules, amylose which helix and amylopectin which is branched
- Since the bonds in starch are α-glucose, the –OH groups from the glucose molecules are always pointed down, causing starch to have
a curved appearance. This makes starch a good molecule for storing glucose in plants. - Even though glucose is hydrophilic, starch is too large to be soluble in water at room temperature
- Cellulose are unbranched straight chains of β (beta) glucose molecules, held together with glycosidic bonds
- Since the –OH groups point out in opposite directions and every other β glucose is flipped 180 degrees, cellulose forms a nice straight
chain - These straight chains also allow cellulose to form bundles linked by H-bonds
- This is essential for cellulose’s function, which is to provide strength for cell walls in plant cells (high tensile strength)
- Notice the up and down alternating glycosidic bonds between the glucose molecules
- Glycogen – Is a multi-branched energy storage polysaccharide for animals
- Glycogen consists of many α (alpha) glucose molecules linked by glycosidic bonds
- It is highly branched, making the molecule more compact and a perfect molecule for energy storage
- It is stored in the liver and some muscles of humans
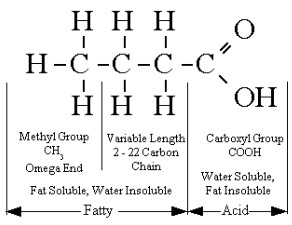
Fatty acid
- Main component of triglycerides(lipids) and phospholipids
- Fatty acids are non-polar and therefore hydrophobic
- Chains consist of covalently bonded carbon with hydrogen
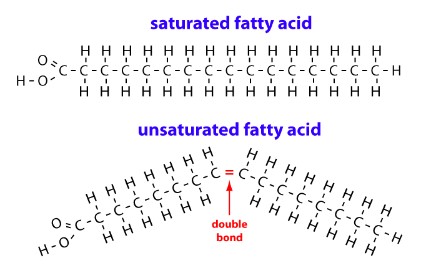
- Saturated FA’s are all single bonds and are therefore saturated with hydrogen.
- Unsaturated FA’s contain a double bond or double bonds
Saturated fatty acid
- Saturated fatty acid are called that because the carbons are carrying as many hydrogen atoms as they can, in other words they are saturated with hydrogen atoms.
- Found in animals products like butter, bacon and red meat
- Solid at room T
- No double bonds between the carbon atoms
Monounsaturated fatty acid
- If one double bond exists in the chain of hydrocarbons, the fatty acid is not saturated. This is monounsaturated
Polyunsaturated fatty acids
- Have at least two double bonds in the carbon chain
- Come from plants (e.g. olive oil)
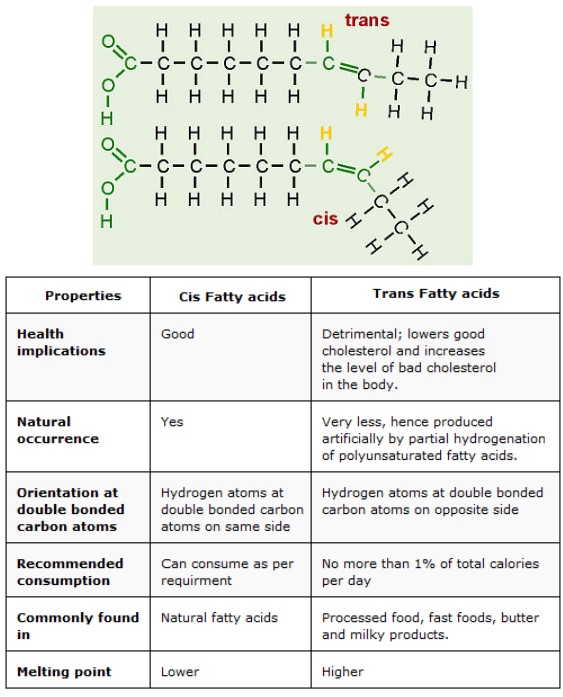
Hydrogenation: cis and trans fatty acids
- If the hydrogen atoms are on the same side of the double bond then the isomer is “cis” and if the hydrogens are on opposite side of the double bond then the isomer is “trans”
- “cis” fatty acids have a bent at the double bonds, causing the fatty acids to pack more loosely, lowering the melting point and making them liquid at room temperature
- “trans” fatty acids do not have the bent at the double bond, can pack more tightly, have a higher melting point and are solid at room temperature.
- Trans fats are partial hydrogenated oils found in some processed foods like margarine. They can cause health risks for humans
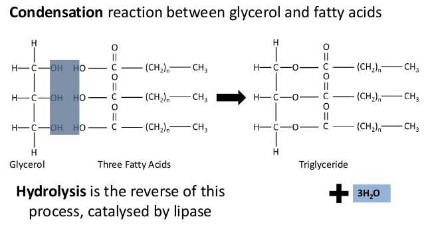
Condensation reactions result in the formation of triglyceride lipids
- Fatty acids have a long hydrocarbon (carbon and hydrogen) chain with a carboxyl (acid) group. The chains usually contain 16 to 18 carbons.
- Glycerol contains 3 carbons and 3 hydroxyl groups. It reacts with 3 fatty acids to form a triglyceride or fat molecule through a condensation reaction, which gives off 3 water molecules and forms and ester bond
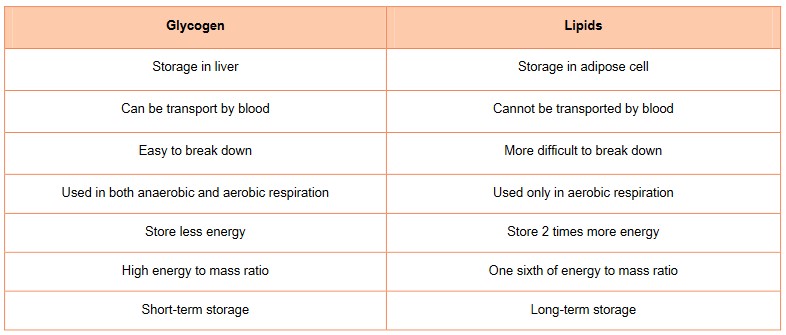
Energy storage
- One’s body requires energy to function, more specifically each cell relies on a source of energy to drive the chemical reactions involved in metabolism, growth and other physiological functions
- Both carbohydrates and lipids (triglycerides) are a major source of energy in animals.
- Fats contain about twice as much energy as carbohydrates. Each gram of carbohydrates stores about 4 calories of energy, whereas each
gram of lipid stores about 9 calories. - Therefore, lipids serve as a more compact way to store energy, since it contains more energy per gram than carbohydrates. As a result, your body tends to use fat to store energy over long periods of time and uses carbohydrates to store energy short-term.
- Glycogen can be quickly into glucose for energy.
- Triglycerides (fats) contain a glycerol and 3 fatty acids and is stored mainly in the body’s adipose tissue
- Fats also provide thermal insulation, protection for organs (shock absorber) and hormones
- Lipids are stored in the adipose cells