Topic : 2 Molecular Biology
2.4 Proteins
Formation of polypeptides
- Cells use the naturally occurring 20 amino acids to synthesize polypeptides.
- When polypeptides are synthesized at ribosomes under the control of genes, the reaction is a condensation reaction
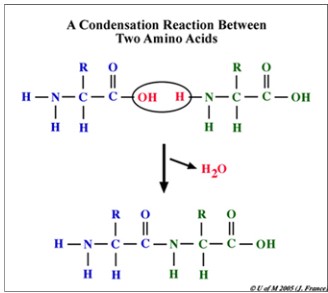
- As there are 20 amino acids, there is a large choice for the sequence of the amino acids as well as total number of amino acids to use within a polypeptide.
- Each polypeptide has own amino acid sequence, own three-dimensional shape
- Polypeptides can contain up to 30,000 amino acids (e.g. Titin) the different possible combinations of polypeptides are effectively infini
Amino acids
- Twenty different amino acids are used by the ribosomes to create polypeptides in our body
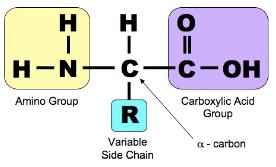
- They all contain an amine (NH2) group, a carboxyl (-COOH) group which combine to form the peptide bond and a “R” group
- The different “R” groups are what makes the amino acids different and allow the proteins to form a wide array of structures and functions
- Some are charged or polar, hence they are hydrophilic
- Some are not charged and are non-polar, hence they are hydrophobic
- In some special cases, R group contains sulfur and selenium
The amino acid sequence of polypeptides is coded for by genes
- Ribosomes need a template – the messenger RNA (mRNA), which is translated by transfer RNA molecules, which carry specific amino acids
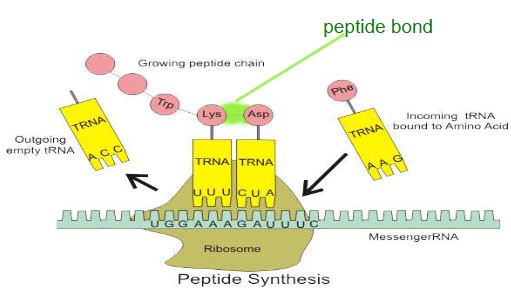
- Genes are simply codes for making polypeptides
- Messenger RNA is a message from the nucleus to the ribosome-instructions for how to put the polypeptide together.
- The genetic code is the sequence of bases on mRNA-this tells the ribosome which amino acids to use
- The sequence of amino acid in polypeptides is coded by the base sequence in an organism’s genes
- Each 3 bases codes for 1 amino acid in a polypeptide(a codon)
- So if a polypeptide is 300 amino acids in length, 900 bases actually code for that polypeptide (not including the 3 base pairs that code for the stop codon). Also, the genes are actually longer as they contain non-coding regions that don’t code for the polypeptide.
- The actual coding region is called the reading frame
Three-dimensional conformation of a protein
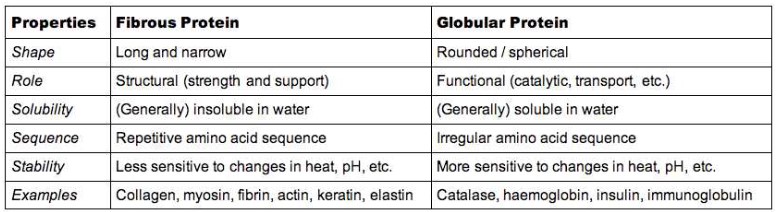
- Proteins are commonly described as either being fibrous or globular in nature.
- Fibrous proteins have structural roles whereas globular proteins are functional (active in a cell’s metabolism).
- Some proteins consist of a single polypeptide, while some contain more than one polypeptide
- Hemoglobin for example has 4 linked polypeptides, which are folded into a globular protein to carry oxygen in the blood
Four levels of protein structure
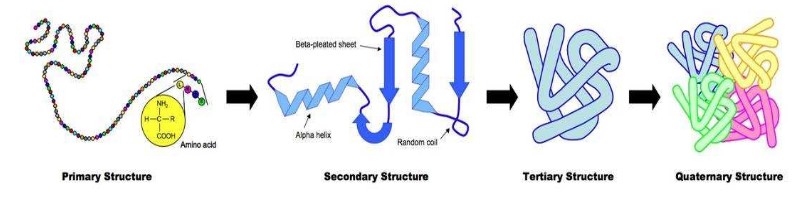
- Primary structure: basic amino acid chain
- Secondary structure: Held together by hydrogen bonds between (non-adjacent) amine (N-H) and carboxylic (C-O) groups, H-bonds provide a level of structural stability. (alpha helix shape & beta pleated sheet)
- Tertiary structure:The polypeptide folds and coils to form a complex 3D shape. Caused by interactions between R groups including ionic
bonds, sulfur bridge, hydrophobic and hydrophilic interaction - Quaternary structure: 2 or more polypeptide chains and/or an inorganic compound (prosthetic group) (e.g.: hemoglobin
Functions of proteins
- Enzyme catalysis
- Muscle contraction
- Cytoskeletons
- Tensile strengthen
- Blood clotting (stop bleeding)
- Transport of nutrients and gases
- Cell adhesion
- Membrane transport
- Hormones
- Receptors
- Packing of DNA
- Immunity
Protein example
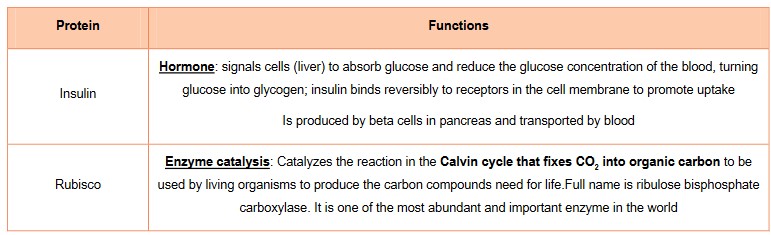
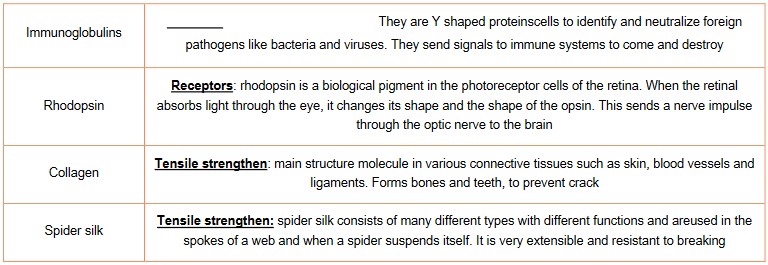
Versatility of proteins
- Biotechnologically has allowed us to use proteins in industry examples are:
1. Enzymes for removing stains in clothing detergent
2. Monoclonal antibodies for pregnancy tests
3. Insulin for treating diabetics
4. Disease treatments
Every individual has a unique proteome
- A proteome is all of the different kinds of proteins produced by a genome, cell, tissue or organism at a certain time.
- This is completed by extracting mixtures of proteins and using gel electrophoresis with antibodies specific to those proteins with florescent markers
- Proteomes vary in different cells (different cells make different proteins) and at different times within the same cell (cell activity varies)
- Proteomes vary between different individuals because of not only cell activity but slight variations in amino acid sequences
- Within species there are strong similarities between proteomes

- The genome determines what proteins an organism can possibly produce. A genome is unique to most individuals (identical twins and
clones share a genome

- Being a function of both the genome and the environment to which the organism is exposed the proteome is both variable (over time)
and unique to every individual (including identical twins and clones). - Environmental factors:influences what proteins an organism needs to produce and in what quantity. (e.g.: nutrition, temperature, activity levels)
- Proteome larger than genome
Denaturation of proteins
- Heat can cause denaturation: vibrations within the molecule break intermolecular bonds or interactions.
- Extremes of pH can cause denaturation: charges on R groups are changed, breaking ionic bonds within the protein or causing new ionic
bonds to form.
