Topic 4 : Ecology
4.3 Carbon cycling
Carbon Cycle
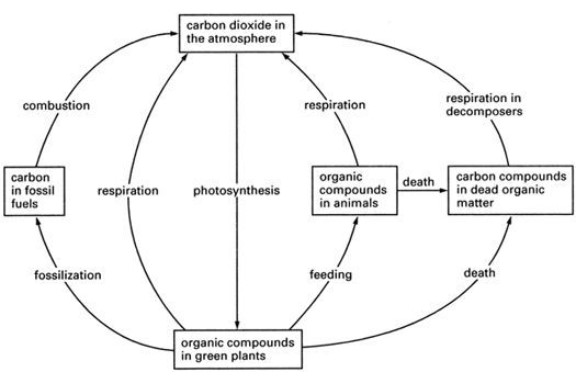
- Autotrophs such as plants and algae, convert inorganic carbon dioxide into organic carbohydrates, lipids and all other carbon based compounds through photosynthesis.
- This reduces the carbon dioxide concentration in the atmosphere.
Carbon in water
- Carbon dioxide dissolves in water and some of it will remain as a dissolved gas
- Some of the carbon dioxide will combine with water to form carbonic acid
\(CO_2 + H_2O <–> H_2CO_3\). - Carbonic acid can then disassociate to form \(H^+\) and \(HCO_3^-\).
\((H_2CO_3 <–>HCO_3^− + H^+)\) - This is why the pH of water decreases
- Autotrophs in water absorb both \(CO_2\) and hydrogen carbonate ions, and use them to produce organic compounds
- Since autotrophs use carbon dioxide for photosynthesis, as the \(CO_2\) is depleted by the autotroph, the concentration of \(CO_2\) in the surrounding atmosphere or water is greater than inside the autotroph; therefore a concentration gradient is created. Carbon dioxide will diffuse from water and air to autotrophs.
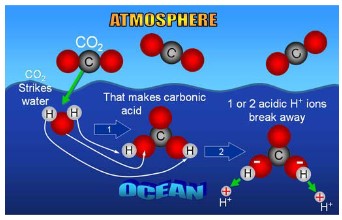
Methanogen
- Archaen microrganisms that produces methane as a metabolic byproduct in anaerobic conditions
such as wetlands, swamps, landfill. - Some bacteria use organic acids and alcohol to produce acetate, carbon dioxide and hydrogen
\(CO_2 + 4 H_2 → CH_4 + 2H_2O\) and \(CH_3COO^− + H^+ → CH_4 + CO_2\) - Methane is the main ingredient in natural gas. When you burn methane the reaction involves oxygen gas from the atmosphere to produce carbon dioxide and water
- When methane is actually released into the atmosphere through the anaerobic reactions, it can persist in the atmosphere for about 12 years, as it is naturally oxidized by monatomic oxygen (O) and hydroxyl radicals (OH–)
- This is why methane concentrations are not very great in the atmosphere, even though large
amounts are produced
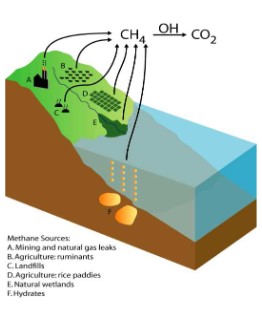
Peat
- Partially decomposed organic matter can be compressed to form brown soil-like peat due to inhibition of decomposers by acidic and anaerobic condition.
- In many soils, saprotrophic bacteria and fungi, digest organic material from dead leaves and plants; however, oxygen (air spaces in the soil) is needed for cellular respiration.
- In muddy, water-logged environments, these air spaces might not be present and therefore anaerobic conditions exist
- Acidic conditions develop, further inhibiting the decomposers
- Since this organic material is not fully decomposed, energy rich molecules that would have been fed upon by saprotrophs and methanogens are left behind and energy rich peat is formed
- Partially decomposed peat when put under extreme weight, pressure and
heat from above sediments can be transformed into coal - This transformation takes place over millions of years
- The dead remains of marine organisms only partial decomposed when they settled at the bottom of the ancient oceans and seas in anaerobic conditions
- As more dead remains and sediment accumulated, intense pressure and heat caused this sludge to undergo a chemical transformation into a mixture of carbon compounds or gases.
- Oil and gas formation occurred in ancient ocean
Formation of limestone
- Animals such as coral and mollusca (clams etc.) contain body parts made out of calcium carbonate \((CaCO3)\)
- Hard corals produce their exoskeletons by secreting calcium carbonate and molluscs have shells that contain calcium carbonate
- The calcium carbonate in alkaline or neutral conditions from a variety of these organisms, settle onto the seafloor when they die
- Through lithification, these sediments form limestone. The hard parts of many of these animals are visible as fossils in the limestone rock
