Topic 8: Metabolism, cell respiration and photosynthesis (HL)
8.1 Metabolism
Metabolic chains and cycles
- Metabolism – the chemical reactions that occur in organisms in order for them to maintain life, such as the synthesis of ATP during cellular respiration.
- In metabolic pathways, enzymes catalyse each reaction along the pathway
- Some of these pathways are anabolic, which is building up of organic molecules (easy to remember as anabolic steroids help build muscle)
- The other pathways are catabolic, which means breaking down of large organic molecules into smaller ones (example – hydrolysis reactions during digestion)
- Some of these metabolic reactions are cycles (i.e. Krebs Cycle) and some are linear chains (i.e. Glycolysis)
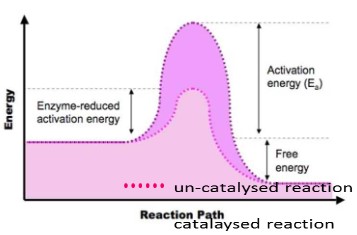
Enzymes lower the activation energy
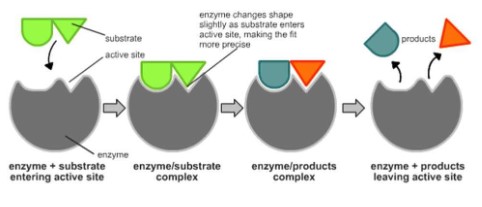
- The substrate binds to the enzymes’ active site and the active site is altered to reach the transition state.
- Due to the binding the bonds in the substrate molecule are stressed/become less stable.
- The binding lowers the overall energy level of the transition state.
- The activation energy of the reaction is therefore reduced.
Notice: the net amount of energy released by the reaction is unchanged - Activation energy is the energy that must be overcome in order for a
chemical reaction to occur. - Activation energy more specifically can be defined as the energy needed to weaken and break the chemical bonds of the substrate.
- Enzymes work by lowering the activation energy needed for the reaction to occur.
- These reactions therefore occur faster and more substrates can be converted into more products (rate of reaction increases dramatically).
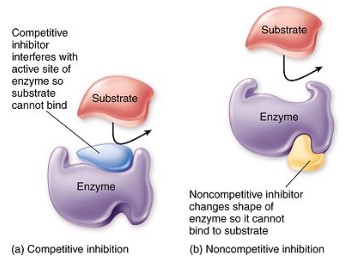
Enzyme inhibitors can be competitive or non-competitive
- Enzyme inhibition occurs when molecules bind to enzymes and decreases their activity.
- Two types of enzyme inhibition are competitive and non-competitive inhibition.
Competitive inhibition
- Competitive inhibition occurs when a molecule that is structurally similar to the substrate competes directly with substrate for access to the active site, thus decreasing the number of times a substrate interacts with an enzyme.
- The inhibitor essentially blocks the substrate from binding to the enzyme.
- Since there is less enzyme/substrate interactions, the chemical reaction rate decreases.
- Competitive inhibition is usually reversible but can be irreversible in some cases.
- Competitive inhibition can be overcome by sufficiently increasing the concentrations of substrate, thereby out-competing the inhibitor.
- With competitive inhibition, the same maximum rate of reaction will be achieved if more substrates are added – because we haven’t changed the number of available enzymes
Non-competitive inhibition
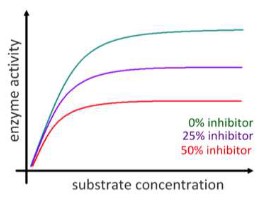
- Non-competitive inhibition occurs when an inhibitor does not compete for the active site with the substrate, but instead binds to a separate site on the enzyme, binding to allosteric site
- When non-competitive inhibitor binds to the enzyme at the alternative site, it changes the conformational shape of the enzyme and thus the active site, so that the substrate can no longer bind to the enzyme for a reaction to occur.
- Non-competitive inhibition is usually reversible.
- Since the inhibitor binds to a site other than the active site, increasing the concentration of the substrate will not speed up the reaction or reduce the effect of the inhibitor
As concentration of non-competitive inhibitor increases, the rate of reaction decreases. This is because there are fewer active sites available for reaction. The maximum rate of reaction is also reduced – with fewer functional active site, the enzyme has reduced ability to process the substrates, even if substrate concentration is increased.
- Antabuse competes with aldehyde oxidase and prevents acetaldehyde from being converted into acetic acid.
- Acetaldehyde builds up and creates a good deterrent from drinking.
- Antabuse is a kind of daily pill, if patient stops taking it, he can drink again.
- Acetaldehyde is a kind of chemical which makes people hangover and sick.
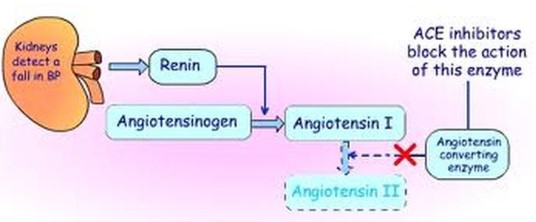
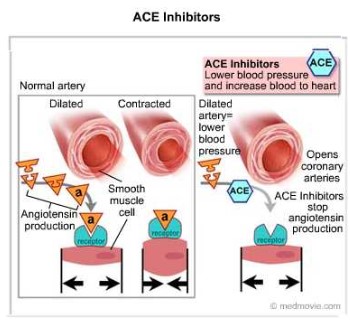
- The RAA system causes vasoconstriction (tightening of blood vessels) when blood pressure drops.
- In people with hypertension or heart failure, the Angiotensin ll can make the condition worse.
- ACE Inhibitors are medications that inhibit Angiotensinogen Converting Enzymes – they prevent increasing blood pressure.
- ACE Inhibitors are non-competitive and reversible.
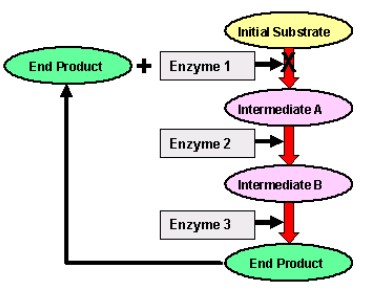
Metabolic pathways can be controlled by end-product inhibition (A.K.A. Feedback Inhibition)
- End-product inhibition prevents the cell from wasting chemical resources and energy by making more of a substance than it needs.
- If a cell is creating a specific product through a metabolic pathway and it makes too much of this product, this product will actually inhibit the first enzyme in the metabolic pathway, thus stopping the metabolic pathway from producing more unneeded product.
- Each step of the reaction is catalysed by a specific enzyme, and a specific end product is present.
- When product is in a sufficient quantity, it inhibits the 1st enzyme and slows down the reaction which means less product made.
- As concentration of product decreases, inhibition decreases and rate of reaction increases.
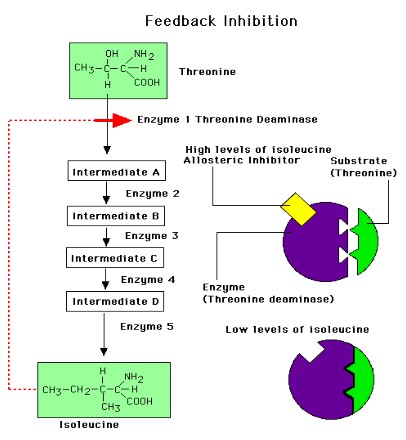
End-product inhibition of the pathway that converts threonine to isoleucine
- Isoleucine is an essential amino acid
- Bacteria synthesize isoleucine from threonine in a series of five enzyme-catalysed steps
- As the concentration of isoleucine increases, some of it binds to the allosteric site of threonine deaminase
- Isoleucine acts as a non-competitive inhibitor to threonine deaminase
- The pathway is then turned off, regulating isoleucine production.
- If the concentration of isoleucine later falls (as a result of its use) then the allosteric sites of threonine deaminase are emptied and the enzymes recommence the conversion of threonine to isoleucine takes place.
Use of databases to identify potential new anti-malarial drugs.
- Bioinformatics is an approach whereby multiple research groups can add information to a database enabling other groups to query the database.
- Bioinformatics has facilitated research into metabolic pathways is referred to as chemogenomics.
- Increasing drug resistance to anti-malarial drugs has lead to the use of bioinformatics and chemogenomics to try and identify new drugs.
- Malaria is a disease caused by the protist Plasmodium falciparum
- The increased resistance of the pathogen P. falciparum to anti-malarial drugs such as chloroquine and the increasing global efforts to eradicate malaria have driven the need to produce new anti-malarial drugs
- P. falciparum strain 3D7 has been sequenced by scientists and is used to test chemicals for new possible medication
- One specific study tested over 300,000 chemicals against a chloroquine-sensitive 3D7 strain and a chloroquine-resistant K1 strain to determine if any of these chemicals inhibited metabolism
- The results showed that 19 new chemicals inhibited the enzymes normally targeted by anti-malarial drugs and 15 chemicals that bound to a total of 61 different malarial proteins.
- This research provides starting points to produce possible new ant-malarial drugs
