Question
A cylinder with a tap contains a fixed mass of gas X. The gas is contained by a piston which can move freely towards or away from the tap.
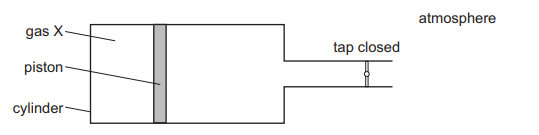
When the tap is opened, the piston moves slightly to the right, towards the tap.
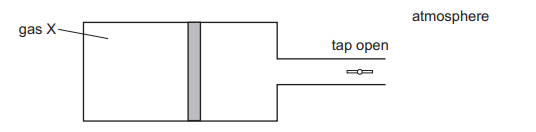
What can be deduced about the pressure of gas X?
| before opening tap | after opening tap |
A | less than atmospheric pressure | more than atmospheric pressure |
B | same as atmospheric pressure | more than atmospheric pressure |
C | more than atmospheric pressure | less than atmospheric pressure |
D | more than atmospheric pressure | same as atmospheric pressure |
Answer/Explanation
Ans: D
The gas is contained by a piston which can move freely towards or away from the tap because there is a more than atmospheric pressure inside the cylinder when a tap is closed.
Question
On a hot day, the pressure of the air in a car tyre is greater than on a cold day.
Why is the pressure greater on a hot day?
The air molecules strike each other more frequently.
The air molecules strike each other with greater force.
The air molecules strike the tyre walls more frequently.
The number of air molecules in the tyre increases.
Answer/Explanation
Ans: C
Question
A gas is compressed in a sealed cylinder by moving a piston.
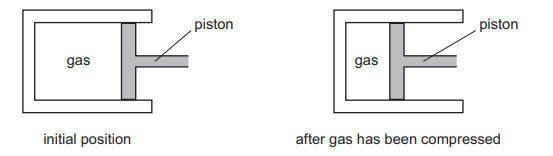
Which row in the table states what happens to the density of the gas and to the pressure of the
gas when it is compressed?
| density | pressure |
A | decreases | decreases |
B | decreases | increases |
C | increases | decreases |
D | increases | increases |
Answer/Explanation
Ans: D
